GALAFOLD- migalastat hydrochloride capsule
Galafold by
Drug Labeling and Warnings
Galafold by is a Prescription medication manufactured, distributed, or labeled by Amicus Therapeutics US, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use GALAFOLD safely and effectively. See full prescribing information for GALAFOLD.
GALAFOLD® (migalastat) capsules, for oral use
Initial U.S. Approval: 2018
INDICATIONS AND USAGE
GALAFOLD is an alpha-galactosidase A (alpha-Gal A) pharmacological chaperone indicated for the treatment of adults with a confirmed diagnosis of Fabry disease and an amenable galactosidase alpha gene (GLA) variant based on in vitro assay data. (1, 12.1)
This indication is approved under accelerated approval based on reduction in kidney interstitial capillary cell globotriaosylceramide (KIC GL-3) substrate. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. (1)
DOSAGE AND ADMINISTRATION
- Select adults with confirmed Fabry disease who have an amenable GLA variant for treatment with GALAFOLD.
- Treatment is indicated for patients with an amenable GLA variant that is interpreted by a clinical genetics professional as causing Fabry disease (pathogenic, likely pathogenic) in the clinical context of the patient. Consultation with a clinical genetics professional is strongly recommended in cases where the amenable GLA variant is of uncertain clinical significance (VUS, variant of uncertain significance) or may be benign (not causing Fabry disease). (2, 12.1)
- The recommended dosage regimen of GALAFOLD is 123 mg orally once every other day at the same time of day. (2)
- Take on an empty stomach. Do not consume food at least 2 hours before and 2 hours after taking GALAFOLD to give a minimum 4 hours fast. (2)
- Do not take GALAFOLD on 2 consecutive days. (2)
- If a dose is missed entirely for the day, take the missed dose only if it is within 12 hours of the normal time that the dose should have been taken. If more than 12 hours have passed, resume taking GALAFOLD at the next planned dosing day and time and according to the every-other-day dosing schedule. (2)
- Swallow capsules whole; do not cut, crush, or chew. (2)
DOSAGE FORMS AND STRENGTHS
Capsules: 123 mg migalastat. (3)
CONTRAINDICATIONS
None. (4)
ADVERSE REACTIONS
Most common adverse drug reactions ≥ 10% are: headache, nasopharyngitis, urinary tract infection, nausea, and pyrexia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amicus Therapeutics at 1-877-4AMICUS or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
GALAFOLD is indicated for the treatment of adults with a confirmed diagnosis of Fabry disease and an amenable galactosidase alpha gene (GLA) variant based on in vitro assay data [see Clinical Pharmacology (12.1)].
This indication is approved under accelerated approval based on reduction in kidney interstitial capillary cell globotriaosylceramide (KIC GL-3) substrate [see Clinical Studies (14)]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
-
2 DOSAGE AND ADMINISTRATION
- Select adults with confirmed Fabry disease who have an amenable GLA variant for treatment with GALAFOLD [see Table 2 in Clinical Pharmacology (12.1)].
- Treatment is indicated for patients with an amenable GLA variant that is interpreted by a clinical genetics professional as causing Fabry disease (pathogenic, likely pathogenic) in the clinical context of the patient. Consultation with a clinical genetics professional is strongly recommended in cases where the amenable GLA variant is of uncertain clinical significance (VUS, variant of uncertain significance) or may be benign (not causing Fabry disease).
- The recommended dosage regimen of GALAFOLD is 123 mg orally once every other day at the same time of day.
- Take GALAFOLD on an empty stomach. Do not consume food at least 2 hours before and 2 hours after taking GALAFOLD to give a minimum 4 hours fast [see Clinical Pharmacology (12.3)]. Clear liquids can be consumed during this 4-hour period.
- Do not take GALAFOLD on 2 consecutive days.
- If a dose is missed entirely for the day, take the missed dose of GALAFOLD only if it is within 12 hours of the normal time that the dose should have been taken. If more than 12 hours have passed, resume taking GALAFOLD at the next planned dosing day and time, according to the every-other-day dosing schedule.
- Swallow capsules whole. Do not cut, crush, or chew.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In clinical trials, 139 patients with Fabry disease (79 females, 60 males, 92% Caucasian, ages 16 to 72 years), who were naïve to GALAFOLD or previously treated with enzyme replacement therapy, were exposed to at least one dose of GALAFOLD. Of the 139 patients, 127 patients were exposed to GALAFOLD 123 mg every other day for 6 months and 123 patients were exposed for greater than one year. The clinical trials included one randomized, double-blind, placebo-controlled clinical trial of 6 months duration followed by a 6-month open-label treatment phase (Study 1) [see Clinical Studies (14)]. A second trial was a randomized, open-label, active-controlled clinical trial of 18 months duration in patients with Fabry disease receiving enzyme replacement therapy who were randomized to either switch to GALAFOLD or continue enzyme replacement therapy (Study 2; NCT01218659). In addition, there were two open-label, long-term extension trials.
The most common adverse reactions reported with GALAFOLD (≥ 10%) during the 6-month placebo-controlled, double-blind phase of Study 1 were headache, nasopharyngitis, urinary tract infection, nausea, and pyrexia.
Table 1 shows adverse reactions reported in at least 5% of patients treated with GALAFOLD (and at a higher rate than placebo) during the 6-month placebo-controlled, double-blind phase of Study 1.
Table 1: Adverse Reactions* Reported During the First 6 Months of Treatment in Patients with Fabry Disease in Study 1 *reported in at least 5% of patients treated with GALAFOLD and at a higher rate than placebo
**included urinary tract infection, cystitis, and kidney infection
Adverse Reaction GALAFOLD
%
(N = 34)Placebo
%
(N = 33)Headache 35% 21% Nasopharyngitis 18% 6% Urinary tract infection** 15% 0 Nausea 12% 6% Pyrexia 12% 3% Abdominal pain 9% 3% Back pain 9% 0 Cough 9% 0 Diarrhea 9% 3% Epistaxis 9% 3% Adverse reactions reported in > 5% of patients who received migalastat in the 6-month open-label treatment phase of Study 1, in Study 2, and in the long-term extension trials (N = 115, mean duration of treatment 2.7 years) included those reported in Table 1 with the addition of vomiting.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Registry
There is a registry that monitors outcomes in individuals with Fabry disease, either exposed or unexposed to GALAFOLD during pregnancy and/or while breastfeeding infants up to 1 year of age. Healthcare providers are encouraged to register patients or obtain additional information by contacting the Pregnancy Coordinating Center at 1-888-239-0758, email at fabrypregnancy@ubc.com, or visit www.fabrypregnancyregistry.com.
Risk Summary
There were three pregnant women with Fabry disease exposed to GALAFOLD in clinical trials. As such, the available data are not sufficient to assess drug associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies, no adverse developmental effects were observed (see Data).
The estimated background risk for major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Animal Data
No adverse developmental effects were observed with oral administration of migalastat to pregnant rats and rabbits during organogenesis at doses up to 26 and 54 times, respectively, the recommended dose based on AUC. No effects on post-natal development were observed following oral administration of up to 500 mg/kg migalastat twice daily to pregnant rats (16 times the recommended dose based on AUC) during organogenesis and through lactation.
8.2 Lactation
Risk Summary
There are no human data available on the presence of migalastat in human milk, the effects on the breastfed infant, or the effects on milk production. Migalastat is present in the milk of lactating rats (see Data). When a drug is present in animal milk, it is likely that the drug will be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for GALAFOLD and any potential adverse effects on the breastfed child from GALAFOLD or from the underlying maternal condition.
Animal Data
Migalastat concentrations in milk from rats following oral administration of up to 500 mg/kg twice daily (approximately 16 times the recommended human dose based on AUC) was approximately 2.5 times higher than levels in the rat maternal plasma at 4 hours post-dose. The concentration of migalastat in plasma from pups was approximately 11 times lower than the maternal plasma concentrations at 1 hour post-dose.
8.3 Females and Males of Reproductive Potential
Infertility
The effects of GALAFOLD on fertility in humans have not been studied. Transient and fully reversible infertility in male rats was associated with migalastat treatment at a systemic exposure (AUC) equivalent to the human exposure at the recommended dose. Complete reversibility was seen at 4 weeks after the termination of treatment. Migalastat did not affect fertility in female rats [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of GALAFOLD have not been established in pediatric patients.
8.5 Geriatric Use
Clinical trials of GALAFOLD did not include a sufficient number of patients 65 years and older to determine whether they respond differently from younger patients.
8.6 Renal Impairment
Migalastat is substantially excreted by the kidneys. Systemic exposure was significantly increased in subjects with severe renal impairment (eGFR less than 30 mL/min/1.73 m2). GALAFOLD has not been studied in patients with Fabry disease who have an eGFR less than 30 mL/min/1.73 m2. GALAFOLD is not recommended for use in patients with severe renal impairment or end-stage renal disease requiring dialysis. No dosage adjustment is required in patients with mild to moderate renal impairment (eGFR at least 30 mL/min/1.73 m2 and above) [see Clinical Pharmacology (12.3)].
-
11 DESCRIPTION
GALAFOLD (migalastat), an alpha-galactosidase A (alpha-Gal A) pharmacological chaperone, contains migalastat hydrochloride as the active ingredient, a low molecular weight iminosugar and an analogue of the terminal galactose of globotriaosylceramide (GL-3).
The chemical name for migalastat hydrochloride is (+)-(2R,3S,4R,5S)-2-(hydroxymethyl) piperidine-3,4,5-triol hydrochloride. Its molecular formula is C6H13NO4HCl, molecular mass is 199.63 g/mol, and its chemical structure is depicted below.
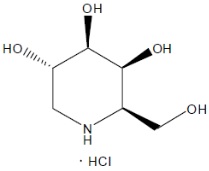
Migalastat hydrochloride is a white to almost white crystalline solid. It is freely soluble in aqueous media within the pH range of 1.2 to 7.5.
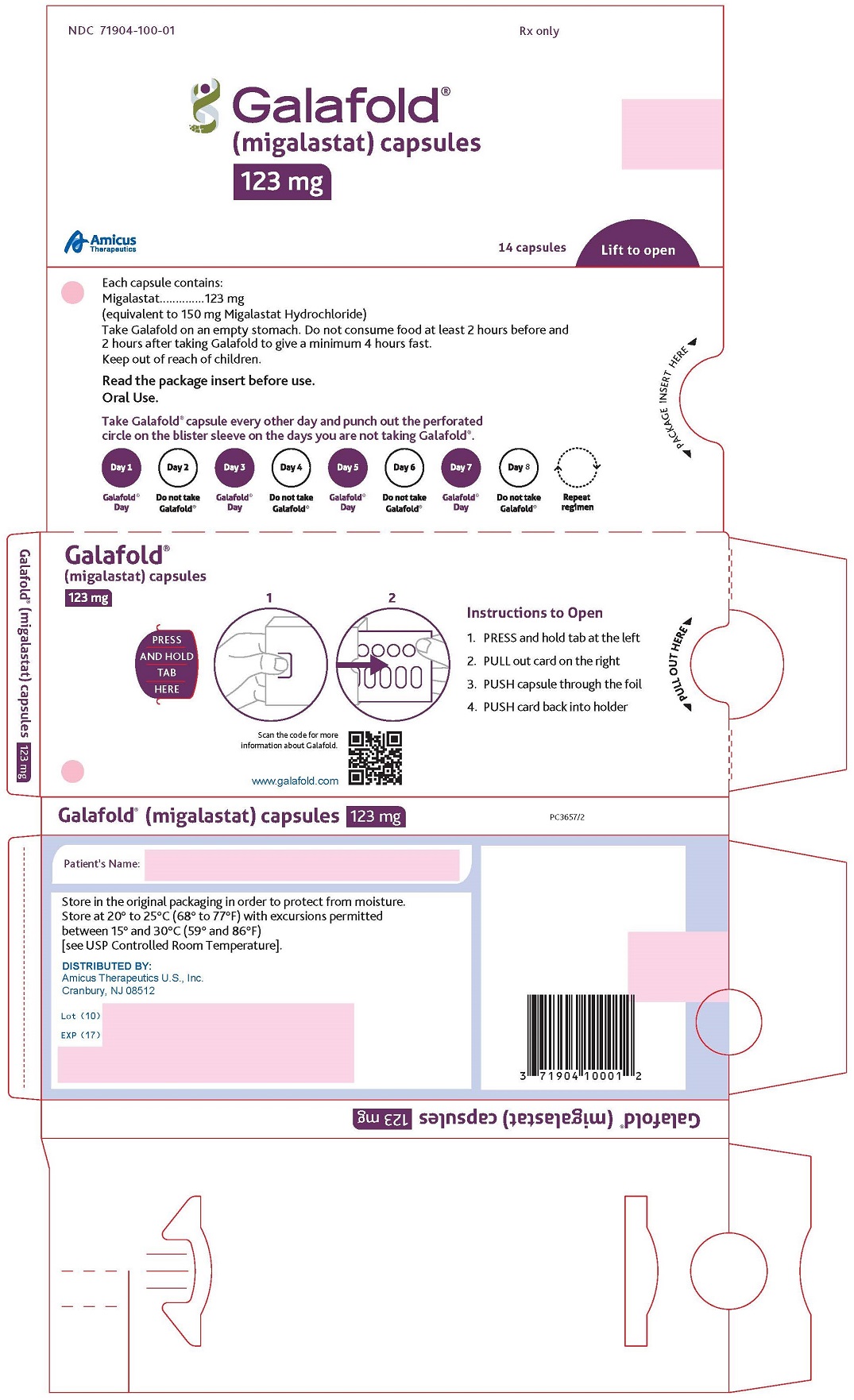
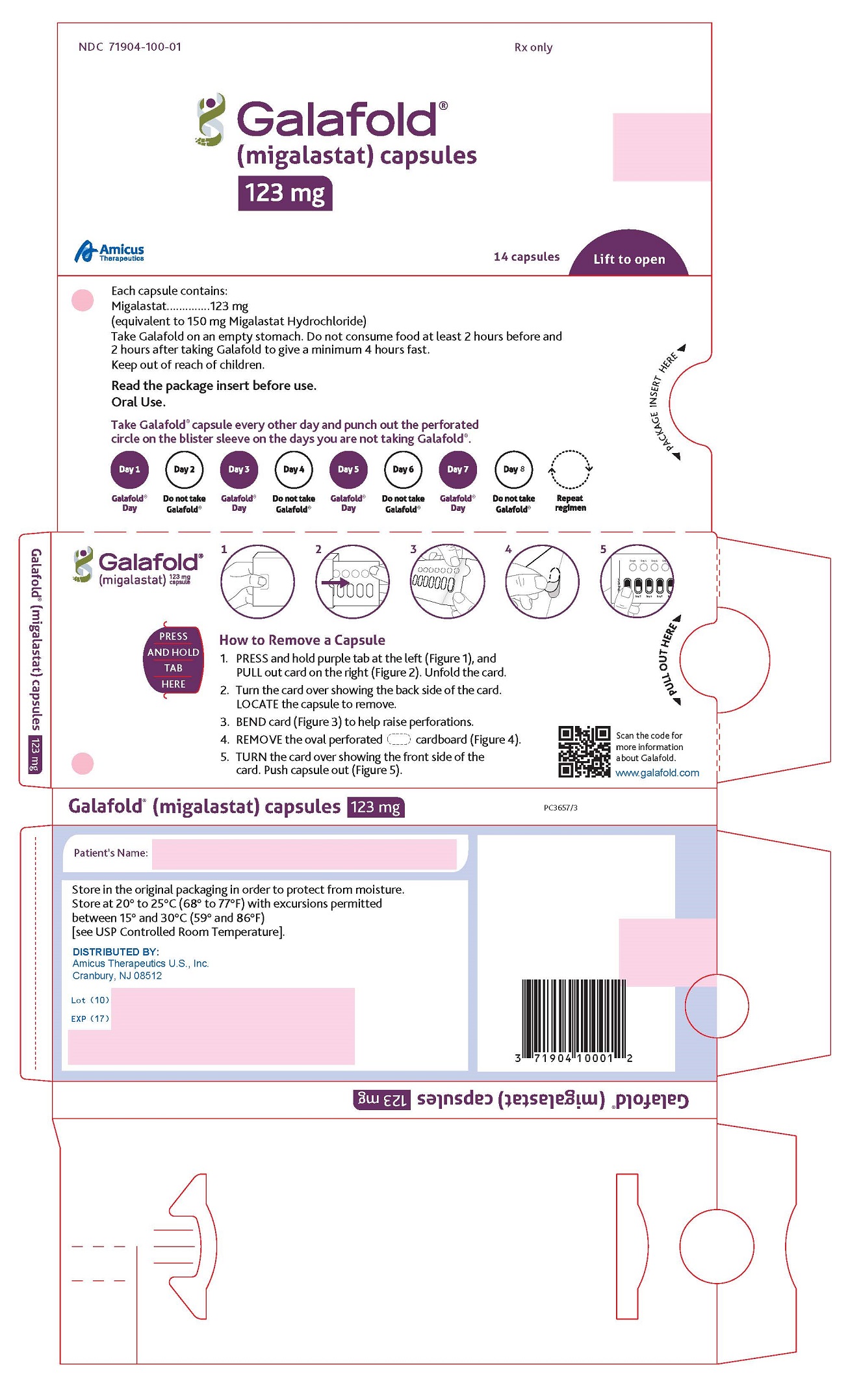
GALAFOLD (migalastat) capsules for oral administration contain 123 mg of migalastat (equivalent to 150 mg migalastat hydrochloride) as a white to pale brown powder and are supplied in a size “2” hard gelatin capsule with an opaque blue cap and an opaque white body imprinted with “A1001” in black ink. The inactive ingredients are magnesium stearate and pregelatinized starch. Capsule shells consist of gelatin, indigotine-FD&C Blue 2, and titanium dioxide. The black ink consists of black iron oxide, potassium hydroxide, and shellac.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Migalastat is a pharmacological chaperone that reversibly binds to the active site of the alpha-galactosidase A (alpha-Gal A) protein (encoded by the galactosidase alpha gene, GLA), which is deficient in Fabry disease. This binding stabilizes alpha-Gal A allowing its trafficking from the endoplasmic reticulum into the lysosome where it exerts its action. In the lysosome, at a lower pH and at a higher concentration of relevant substrates, migalastat dissociates from alpha-Gal A allowing it to break down the glycosphingolipids globotriaosylceramide (GL-3) and globotriaosylsphingosine (lyso-Gb3). Certain GLA variants (mutations) causing Fabry disease result in the production of abnormally folded and less stable forms of the alpha-Gal A protein which, however, retain enzymatic activity. Those GLA variants, referred to as amenable variants, produce alpha-Gal A proteins that may be stabilized by migalastat thereby restoring their trafficking to lysosomes and their intralysosomal activity.
In Vitro Amenability Assay
In an in vitro assay (HEK-293 assay), Human Embryonic Kidney (HEK-293) cell lines were transfected with specific GLA variants (mutations) which produced mutant alpha-Gal A proteins. In the transfected cells, amenability of the GLA variants was assessed after a 5-day incubation with 10 micromol/L migalastat. A GLA variant was categorized as amenable if the resultant mutant alpha-Gal A activity (measured in the cell lysates) met two criteria: 1) it showed a relative increase of at least 20% compared to the pre-treatment alpha-Gal A activity, and 2) it showed an absolute increase of at least 3% of the wild-type (normal) alpha-Gal A activity.
The in vitro assay did not evaluate trafficking of the mutant alpha-Gal A proteins into the lysosome or the dissociation of migalastat from the mutant alpha-Gal A proteins within the lysosome. Also, the in vitro assay did not test whether a GLA variant causes Fabry disease or not.
The GLA variants which are amenable to treatment with GALAFOLD, based on the in vitro assay data, are shown in Table 2. Inclusion of GLA variants in this table does not reflect interpretation of their clinical significance in Fabry disease. Whether a certain amenable GLA variant in a patient with Fabry disease is disease-causing or not should be determined by the prescribing physician (in consultation with a clinical genetics professional, if needed) prior to treatment initiation. Consultation with a clinical genetics professional is strongly recommended in cases where the amenable GLA variant is of uncertain clinical significance (VUS, variant of uncertain significance) or may be benign (not causing Fabry disease).
Table 2: Amenable GLA Variants Based on the In Vitro Assay * Based on available published data, the GLA variant c.937G>T, (p.(D313Y)) is considered benign (not causing Fabry disease). Consultation with a clinical genetics professional is strongly recommended in patients with Fabry disease who have this GLA variant as additional evaluations may be indicated.
DNA Change (Long) DNA Change (Short) Protein Change
(1-letter Code)Protein Change
(3-letter Code)c.7C>G c.C7G p.(L3V) p.(Leu3Val) c.8T>C c.T8C p.(L3P) p.(Leu3Pro) c.[11G>T; 620A>C] c.G11T/A620C p.(R4M/Y207S) p.(Arg4Met/Tyr207Ser) c.37G>A c.G37A p.(A13T) p.(Ala13Thr) c.37G>C c.G37C p.(A13P) p.(Ala13Pro) c.43G>A c.G43A p.(A15T) p.(Ala15Thr) c.44C>G c.C44G p.(A15G) p.(Ala15Gly) c.53T>G c.T53G p.(F18C) p.(Phe18Cys) c.58G>C c.G58C p.(A20P) p.(Ala20Pro) c.59C>A c.C59A p.(A20D) p.(Ala20Asp) c.65T>G c.T65G p.(V22G) p.(Val22Gly) c.70T>C or c.70T>A c.T70C or c.T70A p.(W24R) p.(Trp24Arg) c.70T>G c.T70G p.(W24G) p.(Trp24Gly) c.72G>C or c.72G>T c.G72C or c.G72T p.(W24C) p.(Trp24Cys) c.95T>C c.T95C p.(L32P) p.(Leu32Pro) c.97G>T c.G97T p.(D33Y) p.(Asp33Tyr) c.98A>G c.A98G p.(D33G) p.(Asp33Gly) c.100A>C c.A100C p.(N34H) p.(Asn34His) c.100A>G c.A100G p.(N34D) p.(Asn34Asp) c.101A>C c.A101C p.(N34T) p.(Asn34Thr) c.101A>G c.A101G p.(N34S) p.(Asn34Ser) c.102T>G or c.102T>A c.T102G or c.T102A p.(N34K) p.(Asn34Lys) c.103G>C or c.103G>A c.G103C or c.G103A p.(G35R) p.(Gly35Arg) c.104G>A c.G104A p.(G35E) p.(Gly35Glu) c.104G>T c.G104T p.(G35V) p.(Gly35Val) c.107T>C c.T107C p.(L36S) p.(Leu36Ser) c.107T>G c.T107G p.(L36W) p.(Leu36Trp) c.108G>C or c.108G>T c.G108C or c.G108T p.(L36F) p.(Leu36Phe) c.109G>A c.G109A p.(A37T) p.(Ala37Thr) c.110C>T c.C110T p.(A37V) p.(Ala37Val) c.122C>T c.C122T p.(T41I) p.(Thr41Ile) c.124A>C or c.124A>T c.A124C or c.A124T p.(M42L) p.(Met42Leu) c.124A>G c.A124G p.(M42V) p.(Met42Val) c.125T>A c.T125A p.(M42K) p.(Met42Lys) c.125T>C c.T125C p.(M42T) p.(Met42Thr) c.125T>G c.T125G p.(M42R) p.(Met42Arg) c.126G>A or c.126G>C or c.126G>T c.G126A or c.G126C or c.G126T p.(M42I) p.(Met42Ile) c.137A>C c.A137C p.(H46P) p.(His46Pro) c.142G>C c.G142C p.(E48Q) p.(Glu48Gln) c.152T>A c.T152A p.(M51K) p.(Met51Lys) c.153G>A or c.153G>T or c.153G>C c.G153A or c.G153T or c.G153C p.(M51I) p.(Met51Ile) c.[157A>C; 158A>T] c.A157C/A158T p.(N53L) p.(Asn53Leu) c.157A>G c.A157G p.(N53D) p.(Asn53Asp) c.160C>T c.C160T p.(L54F) p.(Leu54Phe) c.161T>C c.T161C p.(L54P) p.(Leu54Pro) c.164A>G c.A164G p.(D55G) p.(Asp55Gly) c.164A>T c.A164T p.(D55V) p.(Asp55Val) c.[164A>T; 170A>T] c.A164T/A170T p.(D55V/Q57L) p.(Asp55Val/Gln57Leu) c.167G>A c.G167A p.(C56Y) p.(Cys56Tyr) c.167G>T c.G167T p.(C56F) p.(Cys56Phe) c.170A>T c.A170T p.(Q57L) p.(Gln57Leu) c.175G>A c.G175A p.(E59K) p.(Glu59Lys) c.178C>A c.C178A p.(P60T) p.(Pro60Thr) c.178C>T c.C178T p.(P60S) p.(Pro60Ser) c.179C>T c.C179T p.(P60L) p.(Pro60Leu) c.196G>A c.G196A p.(E66K) p.(Glu66Lys) c.197A>G c.A197G p.(E66G) p.(Glu66Gly) c.207C>A or c.207C>G c.C207A or c.C207G p.(F69L) p.(Phe69Leu) c.214A>G c.A214G p.(M72V) p.(Met72Val) c.216G>A or c.216G>T or c.216G>C c.G216A or c.G216T or c.G216C p.(M72I) p.(Met72Ile) c.218C>T c.C218T p.(A73V) p.(Ala73Val) c.227T>C c.T227C p.(M76T) p.(Met76Thr) c.239G>A c.G239A p.(G80D) p.(Gly80Asp) c.239G>T c.G239T p.(G80V) p.(Gly80Val) c.247G>A c.G247A p.(D83N) p.(Asp83Asn) c.253G>A c.G253A p.(G85S) p.(Gly85Ser) c.[253G>A; 254G>A] c.G253A/G254A p.(G85N) p.(Gly85Asn) c.[253G>A; 254G>T; 255T>G] c.G253A/G254T/T255G p.(G85M) p.(Gly85Met) c.254G>A c.G254A p.(G85D) p.(Gly85Asp) c.261G>C or c.261G>T c.G261C or c.G261T p.(E87D) p.(Glu87Asp) c.265C>T c.C265T p.(L89F) p.(Leu89Phe) c.272T>C c.T272C p.(I91T) p.(Ile91Thr) c.288G>A or c.288G>T or c.288G>C c.G288A or c.G288T or c.G288C p.(M96I) p.(Met96Ile) c.289G>C c.G289C p.(A97P) p.(Ala97Pro) c.290C>T c.C290T p.(A97V) p.(Ala97Val) c.305C>T c.C305T p.(S102L) p.(Ser102Leu) c.311G>T c.G311T p.(G104V) p.(Gly104Val) c.316C>T c.C316T p.(L106F) p.(Leu106Phe) c.320A>G c.A320G p.(Q107R) p.(Gln107Arg) c.322G>A c.G322A p.(A108T) p.(Ala108Thr) c.326A>G c.A326G p.(D109G) p.(Asp109Gly) c.334C>G c.C334G p.(R112G) p.(Arg112Gly) c.335G>A c.G335A p.(R112H) p.(Arg112His) c.337T>A c.T337A p.(F113I) p.(Phe113Ile) c.337T>C or c.339T>A or c.339T>G c.T337C or c.T339A or c.T339G p.(F113L) p.(Phe113Leu) c.352C>T c.C352T p.(R118C) p.(Arg118Cys) c.361G>A c.G361A p.(A121T) p.(Ala121Thr) c.368A>G c.A368G p.(Y123C) p.(Tyr123Cys) c.373C>T c.C373T p.(H125Y) p.(His125Tyr) c.374A>T c.A374T p.(H125L) p.(His125Leu) c.376A>G c.A376G p.(S126G) p.(Ser126Gly) c.383G>A c.G383A p.(G128E) p.(Gly128Glu) c.399T>G c.T399G p.(I133M) p.(Ile133Met) c.404C>T c.C404T p.(A135V) p.(Ala135Val) c.408T>A or c.408T>G c.T408A or c.T408G p.(D136E) p.(Asp136Glu) c.416A>G c.A416G p.(N139S) p.(Asn139Ser) c.419A>C c.A419C p.(K140T) p.(Lys140Thr) c.427G>A c.G427A p.(A143T) p.(Ala143Thr) c.431G>A c.G431A p.(G144D) p.(Gly144Asp) c.431G>T c.G431T p.(G144V) p.(Gly144Val) c.434T>C c.T434C p.(F145S) p.(Phe145Ser) c.436C>T c.C436T p.(P146S) p.(Pro146Ser) c.437C>G c.C437G p.(P146R) p.(Pro146Arg) c.454T>C c.T454C p.(Y152H) p.(Tyr152His) c.454T>G c.T454G p.(Y152D) p.(Tyr152Asp) c.455A>G c.A455G p.(Y152C) p.(Tyr152Cys) c.466G>A c.G466A p.(A156T) p.(Ala156Thr) c.466G>T c.G466T p.(A156S) p.(Ala156Ser) c.467C>T c.C467T p.(A156V) p.(Ala156Val) c.471G>C or c.471G>T c.G471C or c.G471T p.(Q157H) p.(Gln157His) c.484T>G c.T484G p.(W162G) p.(Trp162Gly) c.493G>C c.G493C p.(D165H) p.(Asp165His) c.494A>G c.A494G p.(D165G) p.(Asp165Gly) c.496_497delinsTC c.496_497delinsTC p.(L166S) p.(Leu166Ser) c.496C>G c.C496G p.(L166V) p.(Leu166Val) c.[496C>G; 497T>G] c.C496G/T497G p.(L166G) p.(Leu166Gly) c.499C>G c.C499G p.(L167V) p.(Leu167Val) c.506T>C c.T506C p.(F169S) p.(Phe169Ser) c.511G>A c.G511A p.(G171S) p.(Gly171Ser) c.520T>C c.T520C p.(C174R) p.(Cys174Arg) c.520T>G c.T520G p.(C174G) p.(Cys174Gly) c.525C>G or c.525C>A c.C525G or c.C525A p.(D175E) p.(Asp175Glu) c.539T>G c.T539G p.(L180W) p.(Leu180Trp) c.540G>C or c.540G>T c.G540C or c.G540T p.(L180F) p.(Leu180Phe) c.548G>A c.G548A p.(G183D) p.(Gly183Asp) c.548G>C c.G548C p.(G183A) p.(Gly183Ala) c.550T>A c.T550A p.(Y184N) p.(Tyr184Asn) c.551A>G c.A551G p.(Y184C) p.(Tyr184Cys) c.553A>G c.A553G p.(K185E) p.(Lys185Glu) c.559_564dup c.559_564dup p.(M187_S188dup) p.(Met187_Ser188dup) c.559A>G c.A559G p.(M187V) p.(Met187Val) c.560T>C c.T560C p.(M187T) p.(Met187Thr) c.561G>T or c.561G>A or c.561G>C c.G561T or c.G561A or c.G561C p.(M187I) p.(Met187Ile) c.567G>C or c.567G>T c.G567C or c.G567T p.(L189F) p.(Leu189Phe) c.572T>A c.T572A p.(L191Q) p.(Leu191Gln) c.581C>T c.C581T p.(T194I) p.(Thr194Ile) c.584G>T c.G584T p.(G195V) p.(Gly195Val) c.586A>G c.A586G p.(R196G) p.(Arg196Gly) c.593T>C c.T593C p.(I198T) p.(Ile198Thr) c.595G>A c.G595A p.(V199M) p.(Val199Met) c.596T>C c.T596C p.(V199A) p.(Val199Ala) c.596T>G c.T596G p.(V199G) p.(Val199Gly) c.599A>G c.A599G p.(Y200C) p.(Tyr200Cys) c.602C>A c.C602A p.(S201Y) p.(Ser201Tyr) c.602C>T c.C602T p.(S201F) p.(Ser201Phe) c.608A>T c.A608T p.(E203V) p.(Glu203Val) c.609G>C or c.609G>T c.G609C or c.G609T p.(E203D) p.(Glu203Asp) c.611G>T c.G611T p.(W204L) p.(Trp204Leu) c.613C>A c.C613A p.(P205T) p.(Pro205Thr) c.613C>T c.C613T p.(P205S) p.(Pro205Ser) c.614C>T c.C614T p.(P205L) p.(Pro205Leu) c.619T>C c.T619C p.(Y207H) p.(Tyr207His) c.620A>C c.A620C p.(Y207S) p.(Tyr207Ser) c.623T>G c.T623G p.(M208R) p.(Met208Arg) c.628C>T c.C628T p.(P210S) p.(Pro210Ser) c.629C>T c.C629T p.(P210L) p.(Pro210Leu) c.638A>G c.A638G p.(K213R) p.(Lys213Arg) c.638A>T c.A638T p.(K213M) p.(Lys213Met) c.640C>T c.C640T p.(P214S) p.(Pro214Ser) c.641C>T c.C641T p.(P214L) p.(Pro214Leu) c.643A>G c.A643G p.(N215D) p.(Asn215Asp) c.644A>G c.A644G p.(N215S) p.(Asn215Ser) c.[644A>G; 937G>T*] c.A644G/G937T* p.(N215S/D313Y*) p.(Asn215Ser/Asp313Tyr*) c.644A>T c.A644T p.(N215I) p.(Asn215Ile) c.646T>G c.T646G p.(Y216D) p.(Tyr216Asp) c.647A>G c.A647G p.(Y216C) p.(Tyr216Cys) c.655A>C c.A655C p.(I219L) p.(Ile219Leu) c.656T>A c.T656A p.(I219N) p.(Ile219Asn) c.656T>C c.T656C p.(I219T) p.(Ile219Thr) c.659G>A c.G659A p.(R220Q) p.(Arg220Gln) c.659G>C c.G659C p.(R220P) p.(Arg220Pro) c.662A>C c.A662C p.(Q221P) p.(Gln221Pro) c.671A>C c.A671C p.(N224T) p.(Asn224Thr) c.671A>G c.A671G p.(N224S) p.(Asn224Ser) c.673C>G c.C673G p.(H225D) p.(His225Asp) c.683A>G c.A683G p.(N228S) p.(Asn228Ser) c.687T>A or c.687T>G c.T687A or c.T687G p.(F229L) p.(Phe229Leu) c.695T>C c.T695C p.(I232T) p.(Ile232Thr) c.712A>G c.A712G p.(S238G) p.(Ser238Gly) c.713G>A c.G713A p.(S238N) p.(Ser238Asn) c.716T>C c.T716C p.(I239T) p.(Ile239Thr) c.717A>G c.A717G p.(I239M) p.(Ile239Met) c.720G>C or c.720G>T c.G720C or c.G720T p.(K240N) p.(Lys240Asn) c.724A>G c.A724G p.(I242V) p.(Ile242Val) c.724A>T c.A724T p.(I242F) p.(Ile242Phe) c.725T>A c.T725A p.(I242N) p.(Ile242Asn) c.725T>C c.T725C p.(I242T) p.(Ile242Thr) c.728T>G c.T728G p.(L243W) p.(Leu243Trp) c.729G>C or c.729G>T c.G729C or c.G729T p.(L243F) p.(Leu243Phe) c.730G>A c.G730A p.(D244N) p.(Asp244Asn) c.730G>C c.G730C p.(D244H) p.(Asp244His) c.733T>G c.T733G p.(W245G) p.(Trp245Gly) c.740C>G c.C740G p.(S247C) p.(Ser247Cys) c.747C>G or c.747C>A c.C747G or c.C747A p.(N249K) p.(Asn249Lys) c.749A>C c.A749C p.(Q250P) p.(Gln250Pro) c.749A>G c.A749G p.(Q250R) p.(Gln250Arg) c.750G>C c.G750C p.(Q250H) p.(Gln250His) c.758T>C c.T758C p.(I253T) p.(Ile253Thr) c.758T>G c.T758G p.(I253S) p.(Ile253Ser) c.760-762delGTT or c.761-763del c.760_762delGTT or c.761_763del p.(V254del) p.(Val254del) c.769G>C c.G769C p.(A257P) p.(Ala257Pro) c.770C>G c.C770G p.(A257G) p.(Ala257Gly) c.770C>T c.C770T p.(A257V) p.(Ala257Val) c.772G>C or c.772G>A c.G772C or c.G772A p.(G258R) p.(Gly258Arg) c.773G>T c.G773T p.(G258V) p.(Gly258Val) c.776C>A c.C776A p.(P259Q) p.(Pro259Gln) c.776C>G c.C776G p.(P259R) p.(Pro259Arg) c.776C>T c.C776T p.(P259L) p.(Pro259Leu) c.779G>A c.G779A p.(G260E) p.(Gly260Glu) c.779G>C c.G779C p.(G260A) p.(Gly260Ala) c.781G>A c.G781A p.(G261S) p.(Gly261Ser) c.781G>C c.G781C p.(G261R) p.(Gly261Arg) c.781G>T c.G781T p.(G261C) p.(Gly261Cys) c.788A>G c.A788G p.(N263S) p.(Asn263Ser) c.790G>T c.G790T p.(D264Y) p.(Asp264Tyr) c.794C>T c.C794T p.(P265L) p.(Pro265Leu) c.800T>C c.T800C p.(M267T) p.(Met267Thr) c.805G>A c.G805A p.(V269M) p.(Val269Met) c.806T>C c.T806C p.(V269A) p.(Val269Ala) c.809T>C c.T809C p.(I270T) p.(Ile270Thr) c.810T>G c.T810G p.(I270M) p.(Ile270Met) c.811G>A c.G811A p.(G271S) p.(Gly271Ser) c.[811G>A; 937G>T*] c.G811A/G937T* p.(G271S/D313Y*) p.(Gly271Ser/Asp313Tyr*) c.812G>A c.G812A p.(G271D) p.(Gly271Asp) c.823C>G c.C823G p.(L275V) p.(Leu275Val) c.827G>A c.G827A p.(S276N) p.(Ser276Asn) c.829T>G c.T829G p.(W277G) p.(Trp277Gly) c.831G>T or c.831G>C c.G831T or c.G831C p.(W277C) p.(Trp277Cys) c.832A>T c.A832T p.(N278Y) p.(Asn278Tyr) c.835C>G c.C835G p.(Q279E) p.(Gln279Glu) c.838C>A c.C838A p.(Q280K) p.(Gln280Lys) c.840A>T or c.840A>C c.A840T or c.A840C p.(Q280H) p.(Gln280His) c.844A>G c.A844G p.(T282A) p.(Thr282Ala) c.845C>T c.C845T p.(T282I) p.(Thr282Ile) c.850A>G c.A850G p.(M284V) p.(Met284Val) c.851T>C c.T851C p.(M284T) p.(Met284Thr) c.860G>T c.G860T p.(W287L) p.(Trp287Leu) c.862G>C c.G862C p.(A288P) p.(Ala288Pro) c.866T>G c.T866G p.(I289S) p.(Ile289Ser) c.868A>C or c.868A>T c.A868C or c.A868T p.(M290L) p.(Met290Leu) c.869T>C c.T869C p.(M290T) p.(Met290Thr) c.870G>A or c.870G>C or c.870G>T c.G870A or c.G870C or c.G870T p.(M290I) p.(Met290Ile) c.871G>A c.G871A p.(A291T) p.(Ala291Thr) c.877C>A c.C877A p.(P293T) p.(Pro293Thr) c.881T>C c.T881C p.(L294S) p.(Leu294Ser) c.884T>G c.T884G p.(F295C) p.(Phe295Cys) c.886A>G c.A886G p.(M296V) p.(Met296Val) c.886A>T or c.886A>C c.A886T or c.A886C p.(M296L) p.(Met296Leu) c.887T>C c.T887C p.(M296T) p.(Met296Thr) c.888G>A or c.888G>T or c.888G>C c.G888A or c.G888T or c.G888C p.(M296I) p.(Met296Ile) c.893A>G c.A893G p.(N298S) p.(Asn298Ser) c.897C>G or c.897C>A c.C897G or c.C897A p.(D299E) p.(Asp299Glu) c.898C>T c.C898T p.(L300F) p.(Leu300Phe) c.899T>C c.T899C p.(L300P) p.(Leu300Pro) c.901C>G c.C901G p.(R301G) p.(Arg301Gly) c.902G>A c.G902A p.(R301Q) p.(Arg301Gln) c.902G>C c.G902C p.(R301P) p.(Arg301Pro) c.902G>T c.G902T p.(R301L) p.(Arg301Leu) c.907A>T c.A907T p.(I303F) p.(Ile303Phe) c.908T>A c.T908A p.(I303N) p.(Ile303Asn) c.911G>A c.G911A p.(S304N) p.(Ser304Asn) c.911G>C c.G911C p.(S304T) p.(Ser304Thr) c.919G>A c.G919A p.(A307T) p.(Ala307Thr) c.922A>G c.A922G p.(K308E) p.(Lys308Glu) c.924A>T or c.924A>C c.A924T or c.A924C p.(K308N) p.(Lys308Asn) c.925G>C c.G925C p.(A309P) p.(Ala309Pro) c.926C>T c.C926T p.(A309V) p.(Ala309Val) c.928C>T c.C928T p.(L310F) p.(Leu310Phe) c.931C>G c.C931G p.(L311V) p.(Leu311Val) c.935A>G c.A935G p.(Q312R) p.(Gln312Arg) c.936G>T or c.936G>C c.G936T or c.G936C p.(Q312H) p.(Gln312His) c.937G>T* c.G937T* p.(D313Y*) p.(Asp313Tyr*) c.[937G>T*; 1232G>A] c.G937T*/G1232A p.(D313Y*/G411D) p.(Asp313Tyr*/Gly411Asp) c.938A>G c.A938G p.(D313G) p.(Asp313Gly) c.946G>A c.G946A p.(V316I) p.(Val316Ile) c.947T>G c.T947G p.(V316G) p.(Val316Gly) c.950T>C c.T950C p.(I317T) p.(Ile317Thr) c.955A>T c.A955T p.(I319F) p.(Ile319Phe) c.956T>C c.T956C p.(I319T) p.(Ile319Thr) c.958A>C c.A958C p.(N320H) p.(Asn320His) c.959A>T c.A959T p.(N320I) p.(Asn320Ile) c.962A>G c.A962G p.(Q321R) p.(Gln321Arg) c.962A>T c.A962T p.(Q321L) p.(Gln321Leu) c.963G>C or c.963G>T c.G963C or c.G963T p.(Q321H) p.(Gln321His) c.964G>A c.G964A p.(D322N) p.(Asp322Asn) c.964G>C c.G964C p.(D322H) p.(Asp322His) c.966C>A or c.966C>G c.C966A or c.C966G p.(D322E) p.(Asp322Glu) c.967C>A c.C967A p.(P323T) p.(Pro323Thr) c.968C>G c.C968G p.(P323R) p.(Pro323Arg) c.973G>A c.G973A p.(G325S) p.(Gly325Ser) c.973G>C c.G973C p.(G325R) p.(Gly325Arg) c.978G>C or c.978G>T c.G978C or c.G978T p.(K326N) p.(Lys326Asn) c.979C>G c.C979G p.(Q327E) p.(Gln327Glu) c.980A>T c.A980T p.(Q327L) p.(Gln327Leu) c.983G>C c.G983C p.(G328A) p.(Gly328Ala) c.989A>G c.A989G p.(Q330R) p.(Gln330Arg) c.1001G>A c.G1001A p.(G334E) p.(Gly334Glu) c.1010T>C c.T1010C p.(F337S) p.(Phe337Ser) c.1012G>A c.G1012A p.(E338K) p.(Glu338Lys) c.1013A>T c.A1013T p.(E338V) p.(Glu338Val) c.1016T>A c.T1016A p.(V339E) p.(Val339Glu) c.1027C>A c.C1027A p.(P343T) p.(Pro343Thr) c.1028C>T c.C1028T p.(P343L) p.(Pro343Leu) c.1033T>C c.T1033C p.(S345P) p.(Ser345Pro) c.1046G>C c.G1046C p.(W349S) p.(Trp349Ser) c.1055C>G c.C1055G p.(A352G) p.(Ala352Gly) c.1055C>T c.C1055T p.(A352V) p.(Ala352Val) c.1061T>A c.T1061A p.(I354K) p.(Ile354Lys) c.1066C>G c.C1066G p.(R356G) p.(Arg356Gly) c.1066C>T c.C1066T p.(R356W) p.(Arg356Trp) c.1067G>A c.G1067A p.(R356Q) p.(Arg356Gln) c.1067G>C c.G1067C p.(R356P) p.(Arg356Pro) c.1072G>C c.G1072C p.(E358Q) p.(Glu358Gln) c.1073A>C c.A1073C p.(E358A) p.(Glu358Ala) c.1073A>G c.A1073G p.(E358G) p.(Glu358Gly) c.1074G>T or c.1074G>C c.G1074T or c.G1074C p.(E358D) p.(Glu358Asp) c.1076T>C c.T1076C p.(I359T) p.(Ile359Thr) c.1078G>A c.G1078A p.(G360S) p.(Gly360Ser) c.1078G>T c.G1078T p.(G360C) p.(Gly360Cys) c.1079G>A c.G1079A p.(G360D) p.(Gly360Asp) c.1082G>A c.G1082A p.(G361E) p.(Gly361Glu) c.1082G>C c.G1082C p.(G361A) p.(Gly361Ala) c.1084C>A c.C1084A p.(P362T) p.(Pro362Thr) c.1085C>T c.C1085T p.(P362L) p.(Pro362Leu) c.1087C>T c.C1087T p.(R363C) p.(Arg363Cys) c.1088G>A c.G1088A p.(R363H) p.(Arg363His) c.1102G>A c.G1102A p.(A368T) p.(Ala368Thr) c.1117G>A c.G1117A p.(G373S) p.(Gly373Ser) c.1124G>A c.G1124A p.(G375E) p.(Gly375Glu) c.1139C>T c.C1139T p.(P380L) p.(Pro380Leu) c.1153A>G c.A1153G p.(T385A) p.(Tyr385Ala) c.1168G>A c.G1168A p.(V390M) p.(Val390Met) c.1172A>C c.A1172C p.(K391T) p.(Lys391Thr) c.1184G>A c.G1184A p.(G395E) p.(Gly395Glu) c.1184G>C c.G1184C p.(G395A) p.(Gly395Ala) c.1192G>A c.G1192A p.(E398K) p.(Glu398Lys) c.1202_1203insGACTTC c.1202_1203insGACTTC p.(T400_S401dup) p.(Thr400_Ser401dup) c.1208T>C c.T1208C p.(L403S) p.(Leu403Ser) c.1225C>A c.C1225A p.(P409T) p.(Pro409Thr) c.1225C>G c.C1225G p.(P409A) p.(Pro409Ala) c.1225C>T c.C1225T p.(P409S) p.(Pro409Ser) c.1228A>G c.A1228G p.(T410A) p.(Thr410Ala) c.1229C>T c.C1229T p.(T410I) p.(Thr410Ile) c.1232G>A c.G1232A p.(G411D) p.(Gly411Asp) c.1234A>C c.A1234C p.(T412P) p.(Thr412Pro) c.1235C>A c.C1235A p.(T412N) p.(Thr412Asn) c.1253A>G c.A1253G p.(E418G) p.(Glu418Gly) c.1261A>G c.A1261G p.(M421V) p.(Met421Val) If a GLA variant does not appear in Table 2, it is either non-amenable (if tested) or has not been tested for in vitro amenability. For further information, please contact Amicus Medical Information at 1-877-4AMICUS or medinfousa@amicusrx.com.
12.2 Pharmacodynamics
In Study 1, 31 of 50 patients with amenable GLA variants (18 on GALAFOLD, 13 on placebo) had lyso-Gb3 assessments available after 6 months of treatment. The median change from baseline to month 6 in plasma lyso-Gb3 (nmol/L) was -2.37 (range -69.7, 1.8) in patients on GALAFOLD and 0.53 (range -21.5, 16.3) in patients on placebo. In the open-label treatment phase of Study 1, the 13 patients who were initially on placebo for 6 months and who switched to GALAFOLD for another 6 months had a median change in lyso-Gb3 (nmol/L) of -2.72 (range -61.1, -0.3) . The 18 patients who were treated with GALAFOLD for 6 months and then continued GALAFOLD in the open-label treatment phase of Study 1 for an additional 6 months had no further changes in plasma lyso-Gb3.
In Study 2, 46 of 56 patients with amenable GLA variants (31 on GALAFOLD, 15 on enzyme replacement therapy (ERT)) had lyso-Gb3 assessments available after 18 months of treatment. The median change from baseline to month 18 in plasma lyso-Gb3 (nmol/L) was 0.53 (range -2.27, 28.3) in patients on GALAFOLD and -0.03 (range -11.9, 2.57) in patients on ERT.
Cardiac Electrophysiology
At a dose approximately 8 times the recommended dose, GALAFOLD did not prolong the QT interval to any clinically relevant extent.
12.3 Pharmacokinetics
Absorption
Following a single GALAFOLD oral dose of 123 mg, the absolute bioavailability (AUC) of migalastat was approximately 75% and the time to peak plasma concentration was approximately 3 hours. Plasma migalastat exposure (AUC0-∞ and Cmax) demonstrated dose-proportional increases at oral doses from 75 mg to 1250 mg (doses from 0.5 to 8.3-fold of the approved recommended dosage). Migalastat does not accumulate following administration of 123 mg GALAFOLD every other day.
Effect of Food
Administration of GALAFOLD one hour before a high-fat (850 calories; 56% from fat) or light meal (507 calories; 30% from fat), or one hour after a light meal, reduced the mean migalastat AUC0-∞ by 37% to 42% and Cmax by 15% to 39% compared to the fasting state [see Dosage and Administration (2)].
Distribution
The apparent volume of distribution (Vz/F) of migalastat in Fabry patients was approximately 89 L (range: 77 to 133 L) at steady state. There was no detectable plasma protein binding following administration of [14C]-migalastat in the concentration range between 1 to 100 microM.
Elimination
Metabolism
Based upon in vivo data, migalastat is a substrate for uridine diphosphate glucuronosyltransferase (UDPGT), a minor elimination pathway.
Excretion
In a mass balance study in healthy male subjects, following oral administration of 123 mg [14C]-migalastat, approximately 77% of the total radiolabeled dose was recovered in urine and 20% of the total radiolabeled dose was recovered in feces with an overall total recovery of 98% within 96 hours post-dose. In urine, unchanged migalastat accounted for 80% of the radioactivity, which equates to 62% of the administered dose. In feces, unchanged migalastat was the only drug-related component. In plasma, unchanged migalastat accounted for approximately 77% of the plasma radioactivity and three dehydrogenated O-glucuronide conjugated metabolites, M1 to M3, together accounted for approximately 13% of the plasma radioactivity, none of which comprised more than 6% of the radiolabeled dose. Approximately 9% of the total radioactivity in plasma was unassigned.
Following a single oral dose of 123 mg GALAFOLD, migalastat is cleared from plasma with a mean half-life (t½) of approximately 4 hours and apparent clearance of 12.5 L/hr.
Specific Populations
Male and Female Patients: The pharmacokinetic characteristics of migalastat were not significantly different between healthy male and female subjects or patients with Fabry disease.
Racial or Ethnic Groups: Clinical data indicate no ethnic differences in patient populations studied with migalastat.
Patients with Renal Impairment: In a single-dose study in subjects with varying degrees of renal impairment, exposure to migalastat (AUC) was increased by 1.2-, 1.8-, and 4.3-fold in subjects with mild (eGFR 60 to 90 mL/min/1.73 m2), moderate (eGFR 30 to 59 mL/min/1.73 m2), and severe renal impairment (eGFR less than 30 mL/min/1.73 m2), respectively, while the Cmax remained unchanged with severity of renal impairment [see Use in Specific Populations (8.6)].
Drug Interaction Studies
Migalastat is not a known inhibitor or inducer of cytochrome P450 (CYP450) enzymes, nor is it an inhibitor of BCRP, MDR1, P-glycoprotein (P-gp), or BSEP human efflux transporters, or OATP1B1, OATP1B3, OAT1, OAT3, OCT1, OCT2, MATE1, or MATE2-K human uptake transporters. Migalastat is not a substrate of P-gp, BCRP, MDR1 or MATE1, MATE2-K, OAT1, OAT3, or OCT2. Migalastat showed low affinity for SGLT1, as both a substrate and an inhibitor, and showed no activity for SGLT2.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
The carcinogenic potential of migalastat was assessed in a 2-year study in rats and a 26-week study in Tg.rasH2 mice. In the 2-year rat study, migalastat was not tumorigenic at oral doses of up to 600 mg/kg twice daily (24 times the recommended dose based on AUC). In the 26-week study in Tg.rasH2 mice, migalastat was not tumorigenic at oral doses of up to 1000 mg/kg/day in males and 500 mg/kg/day in females.
Mutagenesis
Migalastat was negative in the bacterial mutagenicity (Ames) assay, in vitro cell mutation assay in L5178Y mouse lymphoma TK+/- cells, and in vivo micronucleus assay in rats.
Impairment of Fertility
Oral administration of up to 12.5 mg/kg migalastat twice daily in rats (equivalent to the human AUC at the recommended dose) produced a significant decrease in male fertility. This effect was completely reversed after four weeks of recovery. Female fertility was not affected.
-
14 CLINICAL STUDIES
Study AT1001-011 (referred to as Study 1; NCT00925301) included a 6-month randomized, double-blind, placebo-controlled phase followed by a 6-month open-label treatment phase and a 12-month open-label extension phase. Patients received the recommended dosage of 123 mg GALAFOLD every other day taken without consuming food 2 hours before and 2 hours after each dose to give a minimum 4 hour fast [see Dosage and Administration (2)]. A total of 67 patients with Fabry disease who were naïve to GALAFOLD and enzyme replacement therapy (ERT) or were previously treated with ERT (agalsidase beta or non-U.S. approved agalsidase alfa) and had been off ERT for at least 6 months were randomized in a 1:1 ratio to receive either GALAFOLD 123 mg every other day or placebo for the first 6 months. In the second 6 months, all patients were treated with GALAFOLD. Of the 67 enrolled patients, 50 patients (32 females, 18 males) had amenable GLA variants based on the in vitro amenability assay [see Clinical Pharmacology (12.1)]. The median age of the population was 45 years and 97% were Caucasian. The major efficacy outcome measure of the average number of GL-3 inclusions per kidney interstitial capillary (KIC) in renal biopsy samples was assessed by light microscopy before and after treatment. Efficacy was evaluated after 6 months of treatment in 45 of 50 patients (29 females and 16 males) with available histology data both at baseline and month 6. Of the 45 evaluable patients, 25 received GALAFOLD (18 females, 7 males) and 20 received placebo (11 females, 9 males). The proportion of patients with ≥ 50% reduction from baseline in the average number of GL-3 inclusions per KIC and the median changes from baseline in the average number of GL-3 inclusions per KIC after 6 months of treatment in Study 1 are shown in Table 3.
Table 3: Changes from Baseline to Month 6 in Average Number of GL-3 Inclusions per KIC in Adults with Fabry Disease with Amenable GLA Variants in Study 1 (N = 45) GALAFOLD
n/N (%) with ≥ 50% reduction
Median change from baseline (range)Placebo
n/N (%) with ≥ 50% reduction
Median change from baseline (range)All patients (N = 45) 13/25 (52%)
-0.04 (-1.94, 0.26)9/20 (45%)
-0.03 (-1.00, 1.69)Females (N = 29) 8/18 (44%)
-0.02 (-0.46, 0.26)5/11 (46%)
-0.03 (-0.35, 0.10)Males (N = 16) 5/7 (71%)
-1.10 (-1.94, -0.02)4/9 (44%)
-0.03 (-1.00, 1.69)Patients with baseline
GL-3 ≥ 0.3 (N = 17;
9 males, 8 females)7/9 (78%)
-0.91 (-1.94, 0.19)2/8 (25%)
-0.02 (-1.00, 1.69)Patients with baseline
GL-3 < 0.3 (N = 28;
7 males, 21 females)6/16 (38%)
-0.02 (-0.10, 0.26)7/12 (58%)
-0.05 (-0.16, 0.14)In Study 1, patients with non-amenable GLA variants (n = 17) had no change from baseline in the average number of GL-3 inclusions per KIC after 6 months of treatment.
-
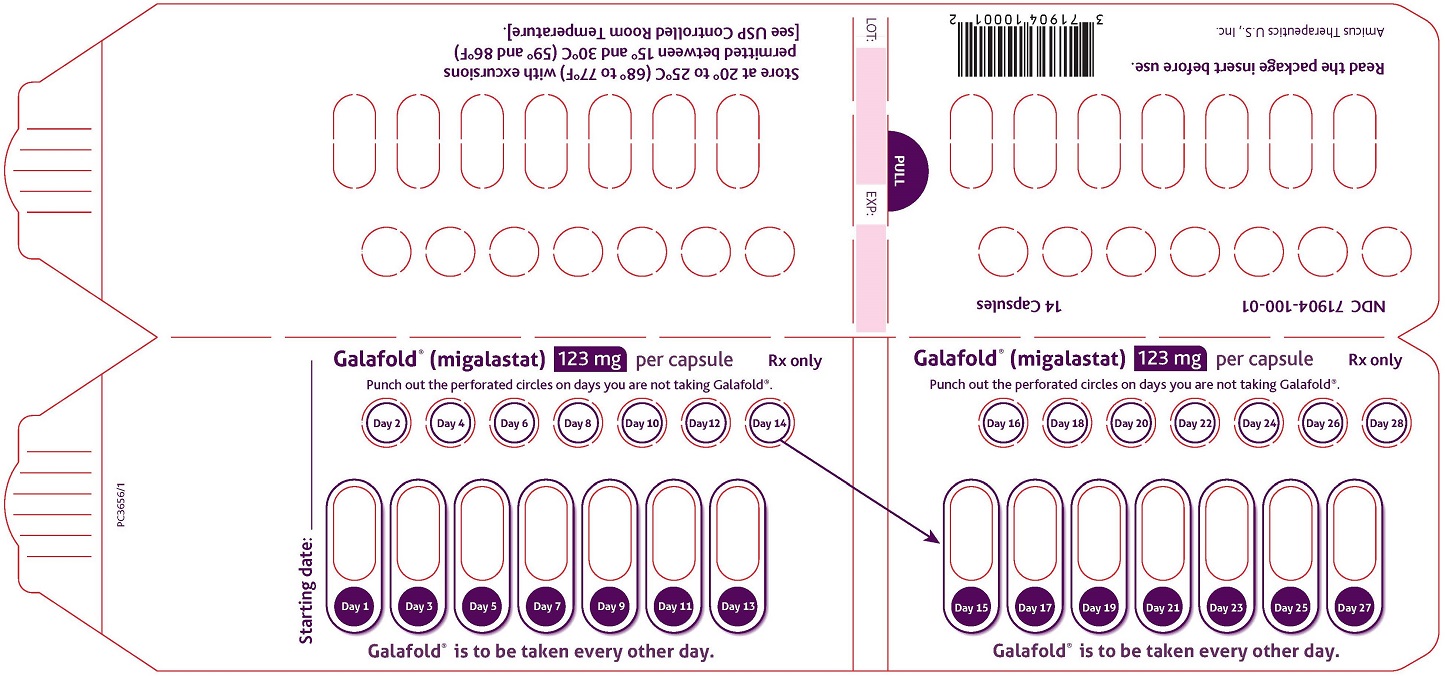
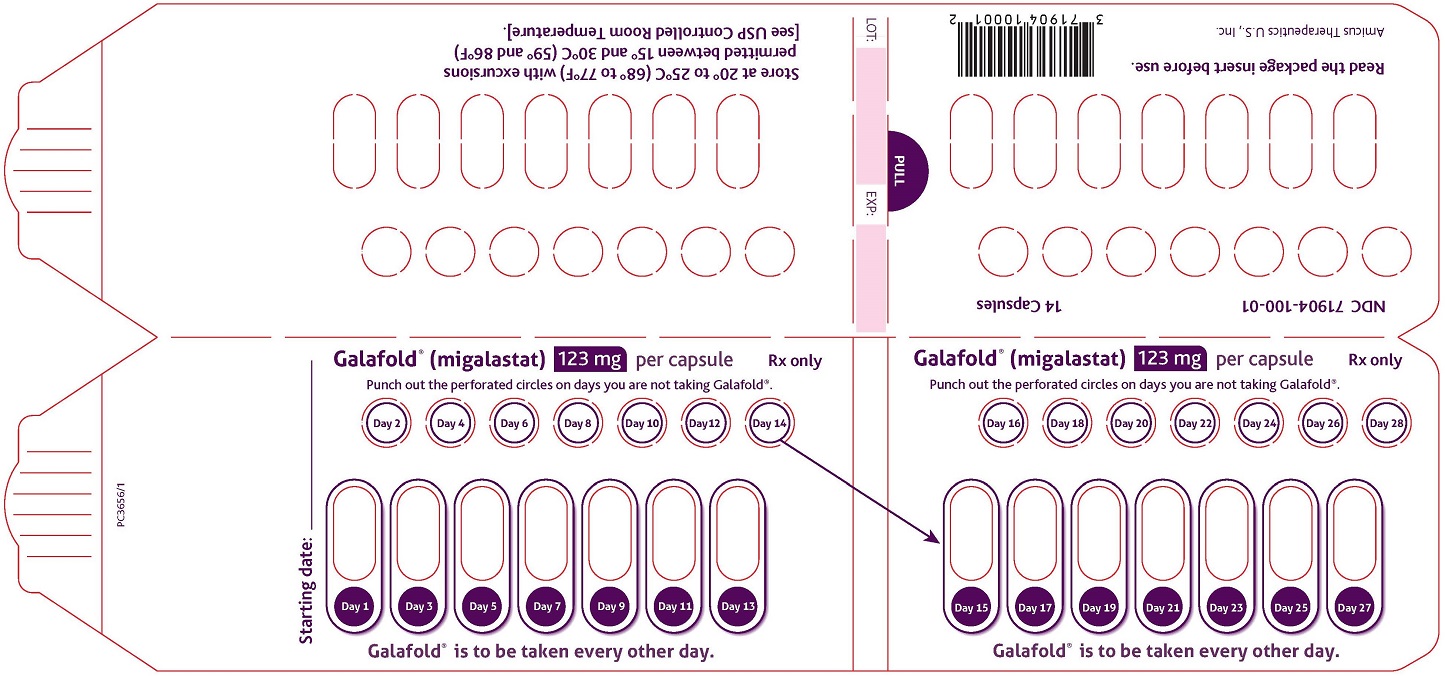
16 HOW SUPPLIED/STORAGE AND HANDLING
GALAFOLD capsules are supplied as 123 mg migalastat, size “2” capsules with opaque blue cap and opaque white body filled with white to pale brown powder and imprinted with “A1001” in black ink.
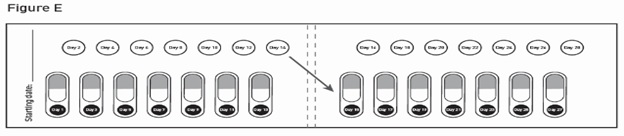
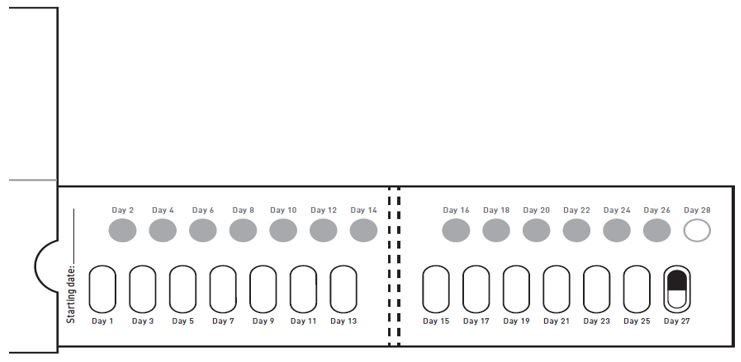
GALAFOLD capsules are packaged as two 7-count capsules blister strips with aluminum foil lidding encased in cardboard blister cards providing 14 capsules per wallet pack that supplies the drug product for 4 weeks (28 days).
Wallet pack containing 14 GALAFOLD capsules NDC: 71904-100-01.
Store at USP Controlled Room Temperature of 20° to 25°C (68° to 77°F) with excursions permitted between 15° and 30°C (59° and 86°F).
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Administration [see Dosage and Administration (2)]:
Advise the patient:
- To take GALAFOLD once every other day at the same time of day.
- Take GALAFOLD on an empty stomach. Do not consume food at least 2 hours before and 2 hours after taking GALAFOLD to give a minimum 4 hours fast. Clear liquids can be consumed during this 4-hour period.
- Not to take GALAFOLD on 2 consecutive days.
- If a dose is missed entirely for the day, take the missed dose only if it is within 12 hours of the normal time that the dose should have been taken. If more than 12 hours have passed, resume taking GALAFOLD at the next planned dosing day and time, according to the every-other-day dosing schedule.
- Swallow capsules whole. Do not cut, crush, or chew.
Pregnancy Registry
Inform patients that there is a registry that monitors outcomes in individuals with Fabry disease, either exposed or unexposed to GALAFOLD during pregnancy and/or while breastfeeding infants up to 1 year of age [see Use in Specific Populations (8.1)].
Manufactured for:
Amicus Therapeutics U.S., Inc.
1 Cedar Brook Drive
Cranbury, NJ 08512GALAFOLD is a registered trademark of Amicus Therapeutics, Inc.
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration.
Issued: March 2020
Patient Information
GALAFOLD® (GAL-a-fold)
(migalastat)
capsulesWhat is GALAFOLD?
GALAFOLD is a prescription medicine used to treat adults with Fabry disease who have a certain genetic change (variant) in the galactosidase alpha gene (GLA) that is responsive (amenable) to GALAFOLD.
It is not known if GALAFOLD is safe and effective in children.Before taking GALAFOLD, tell your healthcare provider about all of your medical conditions, including if you:
- have kidney problems.
- are pregnant or plan to become pregnant. It is not known if GALAFOLD will harm your unborn baby.
If you become pregnant or are breastfeeding an infant up to 1 year of age, talk to your healthcare provider about registering with the pregnancy registry for patients with Fabry disease. The purpose of this registry is to collect information about the safety of this medicine during pregnancy and breastfeeding. To enroll or request additional information, contact the Pregnancy Coordinating Center at 1-888-239-0758, email at fabrypregnancy@ubc.com, or visit www.fabrypregnancyregistry.com. - are breastfeeding or plan to breastfeed. GALAFOLD may pass into breast milk. Talk to your healthcare provider about the best way to feed your baby if you take GALAFOLD.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I take GALAFOLD?
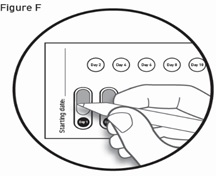
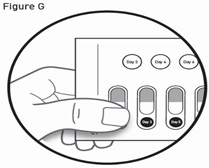
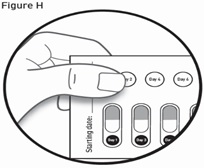
- Read the Instructions for Use at the end of this Patient Information leaflet for detailed instructions about the right way to take GALAFOLD.
- Take 1 GALAFOLD capsule every other day at the same time of day.
- Do not take GALAFOLD two days in a row.
- Take GALAFOLD on an empty stomach. Do not eat at least 2 hours before and 2 hours after taking GALAFOLD. You may drink clear liquids during this 4 hour time when you cannot eat.
- Swallow GALAFOLD capsule whole. Do not cut, crush, or chew the GALAFOLD capsule.
- If you miss a dose of GALAFOLD, take the missed dose of GALAFOLD within 12 hours of your normal schedule. If more than 12 hours have passed, do not make up the missed dose. Take your next dose of GALAFOLD at your next scheduled day and time according to your every-other-day dosing schedule.
- For example, if you miss a dose that you would normally take at 8:00 AM, then you should take that dose before 8:00 PM on the same day. If you do not take the missed dose before 8:00 PM on the same day, you should take your next dose at 8:00 AM on your next scheduled dosing day.
What are the possible side effects of GALAFOLD?
The most common side effects of GALAFOLD include:
- headache
- stuffy or runny nose and sore throat
- urinary tract infection
- nausea
- fever
These are not all the possible side effects of GALAFOLD. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Amicus Therapeutics at 1-877-426-4287.
How should I store GALAFOLD?
- Store GALAFOLD at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep GALAFOLD capsules in the blister card that they come in.
Keep GALAFOLD and all medicines out of the reach of children.
General information about the safe and effective use of GALAFOLD.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use GALAFOLD for a condition for which it was not prescribed. Do not give GALAFOLD to other people, even if they have the same symptoms that you have as it may harm them. You can ask your pharmacist or healthcare provider for information about GALAFOLD that is written for health professionals.
What are the ingredients in GALAFOLD?
Active ingredient: migalastat hydrochloride
Inactive ingredients: magnesium stearate and pregelatinized starch. Capsule shells contain gelatin, indigotine FD&C Blue 2, and titanium dioxide. The black ink contains black iron oxide, potassium hydroxide, and shellac.
Manufactured for: Amicus Therapeutics U.S., Inc., 1 Cedar Brook Drive, Cranbury, NJ 08512
GALAFOLD is a registered trademark of Amicus Therapeutics, Inc.
For more information, go to www.GALAFOLD.com or call 1-877-426-4287.
-
INSTRUCTIONS FOR USE
Instructions for Use
GALAFOLD® (GAL-a-fold)
(migalastat)
capsules
Read this Instructions for Use before you start taking GALAFOLD and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Important information:
- Take 1 GALAFOLD capsule every other day at the same time of day.
- Do not take GALAFOLD two days in a row.
- Take GALAFOLD on an empty stomach. Do not eat at least 2 hours before and 2 hours after taking GALAFOLD. You may drink clear liquids during this 4 hour time when you cannot eat.
- Swallow the GALAFOLD capsule whole. Do not cut, crush, or chew the GALAFOLD capsule.
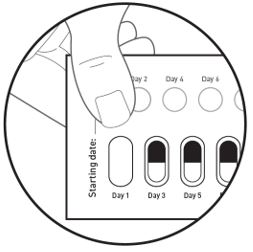
How to remove a capsule:
Replace the blister card back in the carton after each use.
How should I store GALAFOLD?
- Store GALAFOLD at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep GALAFOLD capsules in the blister card they come in.
Keep GALAFOLD and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Manufactured for:
Amicus Therapeutics U.S., Inc.
1 Cedar Brook Drive
Cranbury, NJ 08512GALAFOLD is a registered trademark of Amicus Therapeutics, Inc.
Issued: March 2020
- PRINCIPAL DISPLAY PANEL - NDC: 71904-100-01 - Carton Label (Inner Sleeve)
- PRINCIPAL DISPLAY PANEL - NDC: 71904-100-01 - Carton Label (Outer Sleeve)
-
INGREDIENTS AND APPEARANCE
GALAFOLD
migalastat hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71904-100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MIGALASTAT HYDROCHLORIDE (UNII: CLY7M0XD20) (MIGALASTAT - UNII:C4XNY919FW) MIGALASTAT 123 mg Inactive Ingredients Ingredient Name Strength SHELLAC (UNII: 46N107B71O) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) GELATIN (UNII: 2G86QN327L) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color blue (opaque blue) , white (opaque white) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code A1001 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71904-100-01 14 in 1 CARTON 08/10/2018 1 1 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA208623 08/10/2018 Labeler - Amicus Therapeutics U.S., Inc. (080932337)
Trademark Results [Galafold]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 GALAFOLD 86839473 5680933 Live/Registered |
AMICUS THERAPEUTICS, INC. 2015-12-04 |
 GALAFOLD 86839457 5680931 Live/Registered |
AMICUS THERAPEUTICS, INC. 2015-12-04 |
 GALAFOLD 86465401 5541266 Live/Registered |
Amicus Therapeutics, Inc. 2014-11-26 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.