LORATADINE ALLERGY RELIEF- loratadine tablet
Loratadine Allergy Relief by
Drug Labeling and Warnings
Loratadine Allergy Relief by is a Otc medication manufactured, distributed, or labeled by HEB, Ranbaxy Pharmaceuticals Inc., Ohm Laboratories Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have liver or kidney disease. Your doctor should determine if you need a different dose.
When using this product do not take more than directed. Taking more than directed may cause drowsiness.
- Directions
- Other information
- Inactive ingredients
- Questions?
-
PRINCIPAL DISPLAY PANEL - 10 mg Tablet Blister Pack Carton
Compare to Claritin® active ingredient†
NDC: 37808-529-31
H-E-B®
Allergy Relief
Loratadine Tablets, USP 10 mg
AntihistamineNon-Drowsy* Allergy Relief
Indoor & Outdoor Allergies
24
HourRelief of:
- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Throat or Nose
30 TABLETS
*When taken as directed. See Drug Facts Panel.actual
size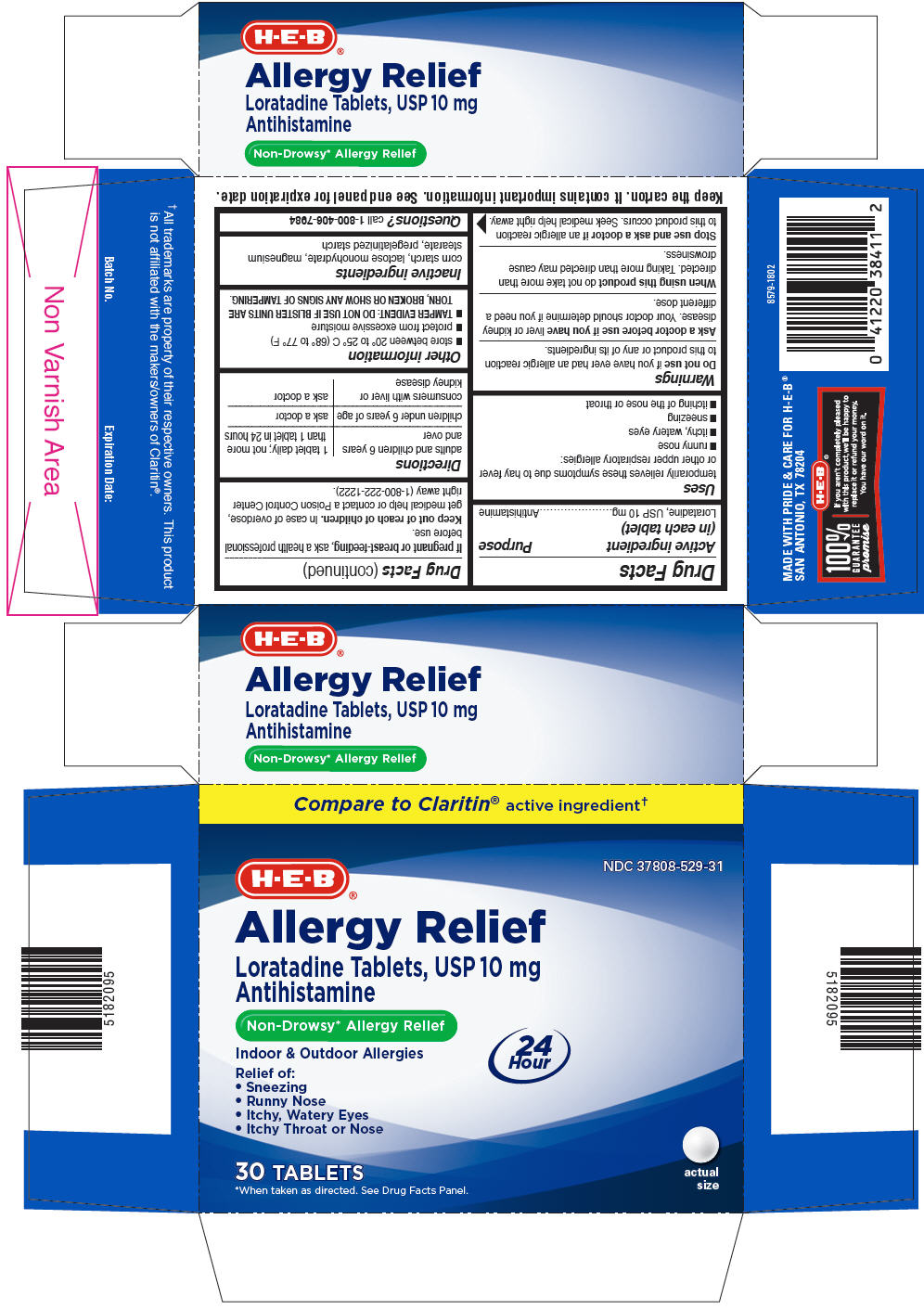
-
INGREDIENTS AND APPEARANCE
LORATADINE ALLERGY RELIEF
loratadine tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 37808-529 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LORATADINE (UNII: 7AJO3BO7QN) (LORATADINE - UNII:7AJO3BO7QN) LORATADINE 10 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) Product Characteristics Color white (White to Off White) Score no score Shape ROUND Size 6mm Flavor Imprint Code RX526 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 37808-529-11 1 in 1 CARTON 02/01/2018 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 37808-529-31 30 in 1 CARTON 02/01/2018 2 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 37808-529-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076134 02/01/2018 Labeler - HEB (007924756) Registrant - Ranbaxy Pharmaceuticals Inc. (937890044) Establishment Name Address ID/FEI Business Operations Ohm Laboratories Inc. 184769029 MANUFACTURE(37808-529)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.