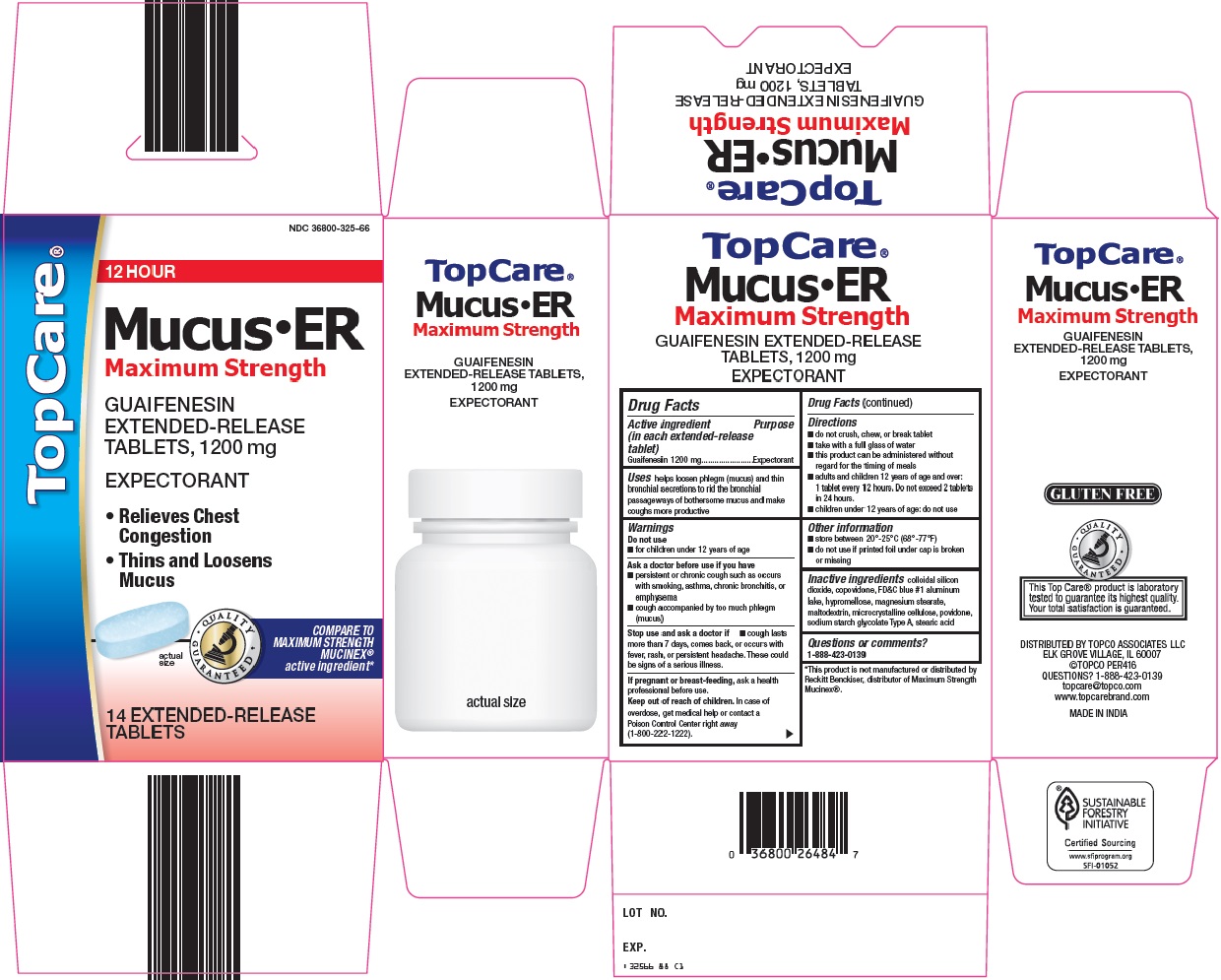
Topco Associates LLC. Mucus ER Drug Facts
TopCare Mucus ER by
Drug Labeling and Warnings
TopCare Mucus ER by is a Otc medication manufactured, distributed, or labeled by Topco Associates LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
TOPCARE MUCUS ER- guaifenesin tablet, extended release
Topco Associates LLC
----------
Topco Associates LLC. Mucus ER Drug Facts
Uses
helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
Warnings
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- cough accompanied by too much phlegm (mucus)
Directions
- do not crush, chew, or break tablet
- take with a full glass of water
- this product can be administered without regard for the timing of meals
- adults and children 12 years of age and over: 1 tablet every 12 hours. Do not exceed 2 tablets in 24 hours.
- children under 12 years of age: do not use
Other information
- store between 20°-25°C (68°-77°F)
- do not use if printed foil under cap is broken or missing
| TOPCARE MUCUS ER
guaifenesin tablet, extended release |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Topco Associates LLC (006935977) |
Revised: 12/2019
Document Id: a36712b3-951f-4045-ba49-a50133c101e8
Set id: 67680619-0dd0-4d29-a103-b9134f0f21ca
Version: 2
Effective Time: 20191216
Topco Associates LLC