LANSOPRAZOLE capsule, delayed release
LANSOPRAZOLE by
Drug Labeling and Warnings
LANSOPRAZOLE by is a Prescription medication manufactured, distributed, or labeled by H.J. Harkins Co., Inc, H.J. Harkins Co.,Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
Indictaions & Usage
1.1 Short-Term Treatment of Active Duodenal Ulcer
Lansoprazole delayed-release capsules are indicated for short-term treatment (for 4 weeks) for healing and symptom relief of active duodenal ulcer. [See CLINICAL STUDIES (14)]
1.2 H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence
Triple Therapy: Lansoprazole delayed-release capsules /amoxicillin /clarithromycin
Lansoprazole delayed-release capsules in combination with amoxicillin plus clarithromycin as triple therapy is indicated for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or one-year history of a duodenal ulcer) to eradicate H. pylori. Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence. [See CLINICAL STUDIES (14)]
Please refer to the full prescribing information for amoxicillin and clarithromycin.
Dual Therapy: Lansoprazole delayed-release capsules /amoxicillin
Dual Therapy: Lansoprazole delayed-release capsules /amoxicillinLansoprazole delayed-release capsules in combination with amoxicillin as dual therapy is indicated for the treatment of patients with H. pylori infection and duodenal ulcer disease (active or one-year history of a duodenal ulcer) who are either allergic or intolerant to clarithromycin or in whom resistance to clarithromycin is known or suspected (see the clarithromycin package insert, MICROBIOLOGY section). Eradication of H. pylori has been shown to reduce the risk of duodenal ulcer recurrence. [See CLINICAL STUDIES (14)]
Please refer to the full prescribing information for amoxicillin.
1.3 Maintenance of Healed Duodenal Ulcers
Lansoprazole delayed-release capsules are indicated to maintain healing of duodenal ulcers. Controlled studies do not extend beyond 12 months. [See CLINICAL STUDIES (14)]
1.4 Short-Term Treatment of Active Benign Gastric Ulcer
Lansoprazole delayed-release capsules are indicated for short-term treatment (up to 8 weeks) for healing and symptom relief of active benign gastric ulcer. [See CLINICAL STUDIES (14)]
1.5 Healing of NSAID-Associated Gastric Ulcer
Lansoprazole delayed-release capsules are indicated for the treatment of NSAID-associated gastric ulcer in patients who continue NSAID use. Controlled studies did not extend beyond 8 weeks. [See CLINICAL STUDIES (14)]
1.6 Risk Reduction of NSAID-Associated Gastric Ulcer
Lansoprazole delayed-release capsules are indicated for reducing the risk of NSAID-associated gastric ulcers in patients with a history of a documented gastric ulcer who require the use of an NSAID. Controlled studies did not extend beyond 12 weeks. [See CLINICAL STUDIES (14)]
1.7 Gastroesophageal Reflux Disease (GERD)
Short-Term Treatment of Symptomatic GERD
Lansoprazole delayed-release capsules is indicated for the treatment of heartburn and other symptoms associated with GERD for upto 8 weeks. [See CLINICAL STUDIES (14)]
Short-Term Treatment of Erosive Esophagitis
Lansoprazole delayed-release capsules is indicated for short-term treatment (up to 8 weeks) for healing and symptom relief of all grades of erosive esophagitis.
For patients who do not heal with lansoprazole delayed-release capsules for 8 weeks (5 to10%), it may be helpful to give an additional 8 weeks of treatment. If there is a recurrence of erosive esophagitis an additional 8-week course of lansoprazole delayed-release capsules may be considered. [See CLINICAL STUDIES (14)]
1.8 Maintenance of Healing of Erosive Esophagitis (EE)
Lansoprazole delayed-release capsules are indicated to maintain healing of erosive esophagitis. Controlled studies did not extend beyond 12 months. [See CLINICAL STUDIES (14)]
1.9 Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome (ZES)
Lansoprazole delayed-release capsules are indicated for the long-term treatment of pathological hypersecretory conditions, including Zollinger-Ellison syndrome. [See CLINICAL STUDIES (14)]
-
Dosage Forms & Strengths
Lansoprazole delayed release capsules USP, 15 mg are white to pale yellow colored enteric coated pellets filled in size ‘3’ hard gelatin capsules with opaque pink colored cap and opaque green colored body, imprinted ‘RDY’ on cap and ‘LAN’ on body with white ink.
Lansoprazole delayed release capsules USP, 30 mg are white to pale yellow colored enteric coated pellets filled in size ‘1’ hard gelatin capsules with opaque pink colored cap and opaque black colored body, imprinted ‘RDY’ on cap and ‘399’ on body with white ink. -
Contraindications
Lansoprazole delayed-release capsules are contraindicated in patients with known severe hypersensitivity to any component of the formulation of lansoprazole delayed-release capsules. Hypersensitivity reactions may include anaphylaxis, anaphylactic shock, angioedema, bronchospasm, acute interstitial nephritis, and urticaria [see Adverse Reactions (6)].
-
Warnings & Precautions
5.1 Gastric Malignancy
Symptomatic response to therapy with lansoprazole does not preclude the presence of gastric malignancy.
5.2 Acute Interstitial Nephritis
Acute interstitial nephritis has been observed in patients taking PPIs including lansoprazole. Acute interstitial nephritis may occur at any point during PPI therapy and is generally attributed to an idiopathic hypersensitivity reaction. Discontinue lansoprazole delayed-release capsules if acute interstitial nephritis develops [see Contraindications (4)].
5.3 Cyanocobalamin (vitamin B12) Deficiency
Daily treatment with any acid-suppressing medications over a long period of time (e.g., longer than 3 years) may lead to malabsorption of cyanocobalamin (vitamin B12) caused by hypo- or achlorhydria. Rare reports of cyanocobalamin deficiency occurring with acid-suppressing therapy have been reported in the literature. This diagnosis should be considered if clinical symptoms consistent with cyanocobalamin deficiency are observed.
5.4 Clostridium difficile Associated Diarrhea
Published observational studies suggest that proton pump inhibitor (PPI) therapy like lansoprazole delayed-release capsules may be associated with an increased risk of Clostridium difficile associated diarrhea (CDAD), especially in hospitalized patients. This diagnosis should be considered for diarrhea that does not improve [see Adverse Reactions (6.2)].
Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated.
CDAD has been reported with use of nearly all antibacterial agents. For more information specific to antibacterial agents (clarithromycin and amoxicillin) indicated for use in combination with lansoprazole delayed release capsules, refer to WARNINGS and PRECAUTIONS sections of those package inserts.
5.5 Bone Fracture
Several published observational studies suggest that PPI therapy may be associated with an increased risk for osteoporosis-related fractures of the hip, wrist or spine. The risk of fracture was increased in patients who received high-dose, defined as multiple daily doses, and long-term PPI therapy (a year or longer). Patients should use the lowest dose and shortest duration of PPI therapy appropriate to the condition being treated. Patients at risk for osteoporosis-related fractures should be managed according to established treatment guidelines [see DOSAGE AND ADMINISTRATION (2) and ADVERSE REACTIONS (6.2)].
5.6 Hypomagnesemia
Hypomagnesemia, symptomatic and asymptomatic, has been reported rarely in patients treated with PPIs for at least three months, in most cases after a year of therapy. Serious adverse events include tetany, arrhythmias, and seizures. In most patients, treatment of hypomagnesemia required magnesium replacement and discontinuation of the PPI.
For patients expected to be on prolonged treatment or who take PPIs with medications such as digoxin or drugs that may cause hypomagnesemia (e.g., diuretics), health care professionals may consider monitoring magnesium levels prior to initiation of PPI treatment and periodically [see Adverse Reactions (6.2)].
5.7 Concomitant use of lansoprazole delayed-release capsules with Methotrexate
Literature suggests that concomitant use of PPIs with methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. In high-dose methotrexate administration, a temporary withdrawal of the PPI may be considered in some patients [see Drug Interactions (7.6)and Clinical Pharmacology (12.3)].
-
Adverse Reactions
6.1 Clinical
Worldwide, over 10,000 patients have been treated with lansoprazole delayed-release capsules in Phase 2 or Phase 3 clinical trials involving various dosages and durations of treatment. In general, lansoprazole delayed-release capsules treatment has been well-tolerated in both short-term and long-term trials.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The following adverse reactions were reported by the treating physician to have a possible or probable relationship to drug in 1% or more of lansoprazole delayed-release capsules-treated patients and occurred at a greater rate in lansoprazole delayed-release capsules-treated patients than placebo-treated patients in Table 1.
Table 1: Incidence of Possibly or Probably Treatment-Related Adverse Reactions in Short-Term, Placebo-Controlled lansoprazole delayed-release capsules Studies
Body System/Adverse Event. lansoprazole delayed-release capsules Placebo (N= 2768) (n=2768) % (n=1023) %
Body as a Whole
Abdominal Pain 2.1 1.2
Digestive System
Constipation 1 0 .4
Diarrhea 3.8 2.3
Nausea 1.3 1.2Headache was also seen at greater than 1% incidence but was more common on placebo. The incidence of diarrhea was similar between patients who received placebo and patients who received 15 mg and 30 mg of lansoprazole delayed-release capsules, but higher in the patients who received 60 mg of lansoprazole delayed-release capsules (2.9%, 1.4%, 4.2%, and 7.4%, respectively).
The most commonly reported possibly or probably treatment-related adverse event during maintenance therapy was diarrhea.
In the risk reduction study of lansoprazole delayed-release capsules for NSAID-associated gastric ulcers, the incidence of diarrhea for patients treated with lansoprazole delayed-release capsules, misoprostol, and placebo was 5%, 22%, and 3%, respectively.
Another study for the same indication, where patients took either a COX-2 inhibitor or lansoprazole and naproxen, demonstrated that the safety profile was similar to the prior study. Additional reactions from this study not previously observed in other clinical trials with lansoprazole delayed-release capsules included contusion, duodenitis, epigastric discomfort, esophageal disorder, fatigue, hunger, hiatal hernia, hoarseness, impaired gastric emptying, metaplasia, and renal impairment.
Additional adverse experiences occurring in less than 1% of patients or subjects who received lansoprazole delayed-release capsules in domestic trials are shown below:
Body as a Whole – abdomen enlarged, allergic reaction, asthenia, back pain, candidiasis, carcinoma, chest pain (not otherwise specified), chills, edema, fever, flu syndrome, halitosis, infection (not otherwise specified), malaise, neck pain, neck rigidity, pain, pelvic pain
Cardiovascular System - angina, arrhythmia, bradycardia, cerebrovascular accident/cerebral infarction, hypertension/hypotension, migraine, myocardial infarction, palpitations, shock (circulatory failure), syncope, tachycardia, vasodilation
Digestive System – abnormal stools, anorexia, bezoar, cardiospasm, cholelithiasis, colitis, dry mouth, dyspepsia, dysphagia, enteritis, eructation, esophageal stenosis, esophageal ulcer, esophagitis, fecal discoloration, flatulence, gastric nodules/fundic gland polyps, gastritis, gastroenteritis, gastrointestinal anomaly, gastrointestinal disorder, gastrointestinal hemorrhage, glossitis, gum hemorrhage, hematemesis, increased appetite, increased salivation, melena, mouth ulceration, nausea and vomiting, nausea and vomiting and diarrhea, gastrointestinal moniliasis, rectal disorder, rectal hemorrhage, stomatitis, tenesmus, thirst, tongue disorder, ulcerative colitis, ulcerative stomatitis
Endocrine System - diabetes mellitus, goiter, hypothyroidism
Hemic and Lymphatic System - anemia, hemolysis, lymphadenopathy
Metabolism and Nutritional Disorders - avitaminosis, gout, dehydration, hyperglycemia/hypoglycemia, peripheral edema, weight gain/loss
Musculoskeletal System - arthralgia, arthritis, bone disorder, joint disorder, leg cramps, musculoskeletal pain, myalgia, myasthenia, ptosis, synovitis
Nervous System – abnormal dreams, agitation, amnesia, anxiety, apathy, confusion, convulsion, dementia, depersonalization, depression, diplopia, dizziness, emotional lability, hallucinations, hemiplegia, hostility aggravated, hyperkinesia, hypertonia, hypesthesia, insomnia, libido decreased/increased, nervousness, neurosis, paresthesia, sleep disorder, somnolence, thinking abnormality, tremor, vertigo
Respiratory System - asthma, bronchitis, cough increased, dyspnea, epistaxis, hemoptysis, hiccup, laryngeal neoplasia, lung fibrosis, pharyngitis, pleural disorder, pneumonia, respiratory disorder, upper respiratory inflammation/infection, rhinitis, sinusitis, stridor
Skin and Appendages - acne, alopecia, contact dermatitis, dry skin, fixed eruption, hair disorder, maculopapular rash, nail disorder, pruritus, rash, skin carcinoma, skin disorder, sweating, urticaria
Special Senses – abnormal vision, amblyopia, blepharitis, blurred vision, cataract, conjunctivitis, deafness, dry eyes, ear/eye disorder, eye pain, glaucoma, otitis media, parosmia, photophobia, retinal degeneration/disorder, taste loss, taste perversion, tinnitus, visual field defect
Urogenital System - abnormal menses, breast enlargement, breast pain, breast tenderness, dysmenorrhea, dysuria, gynecomastia, impotence, kidney calculus, kidney pain, leukorrhea, menorrhagia, menstrual disorder, penis disorder, polyuria, testis disorder, urethral pain, urinary frequency, urinary retention, urinary tract infection, urinary urgency, urination impaired, vaginitis.
6.2 Postmarketing Experience
Additional adverse experiences have been reported since lansoprazole delayed-release capsules has been marketed. The majority of these cases are foreign-sourced and a relationship to lansoprazole delayed-release capsules has not been established. Because these reactions were reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events are listed below by COSTART body system.
Body as a Whole – anaphylactic/anaphylactoid reactions; Digestive System - hepatotoxicity, pancreatitis, vomiting; Hemic and Lymphatic System - agranulocytosis, aplastic anemia, hemolytic anemia, leukopenia, neutropenia, pancytopenia, thrombocytopenia, and thrombotic thrombocytopenic purpura; Infections and Infestations – Clostridium difficile associated diarrhea; Metabolism and Nutritional Disorders – hypomagnesemia; Musculoskeletal System - bone fracture,myositis; Skin and Appendages – severe dermatologic reactions including erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis (some fatal); Special Senses - speech disorder; Urogenital System – interstitial nephritis, urinary retention.
6.3 Combination Therapy with Amoxicillin and Clarithromycin
In clinical trials using combination therapy with lansoprazole delayed-release capsules plus amoxicillin and clarithromycin, and lansoprazole delayed-release capsules plus amoxicillin, no adverse reactions peculiar to these drug combinations were observed. Adverse reactions that have occurred have been limited to those that had been previously reported with lansoprazole delayed-release capsules, amoxicillin, or clarithromycin.
Triple Therapy: Lansoprazole delayed-release capsules /amoxicillin/clarithromycin
The most frequently reported adverse reactions for patients who received triple therapy for 14 days were diarrhea (7%), headache (6%), and taste perversion (5%). There were no statistically significant differences in the frequency of reported adverse reactions between the 10 and 14 day triple therapy regimens. No treatment-emergent adverse reactions were observed at significantly higher rates with triple therapy than with any dual therapy regimen.
Dual Therapy: Lansoprazole delayed-release capsules /amoxicillin
The most frequently reported adverse reactions for patients who received lansoprazole delayed-release capsules three times daily plus amoxicillin three times daily dual therapy were diarrhea (8%) and headache (7%). No treatment-emergent adverse reactions were observed at significantly higher rates with lansoprazole delayed-release capsules three times daily plus amoxicillin three times daily dual therapy than with lansoprazole delayed-release capsules alone.
For information about adverse reactions with antibacterial agents (amoxicillin and clarithromycin) indicated in combination with lansoprazole delayed-release capsules, refer to the ADVERSE REACTIONS section of their package inserts.
6.4 Laboratory Values
The following changes in laboratory parameters in patients who received lansoprazole delayed-release capsules were reported as adverse reactions:
Abnormal liver function tests, increased SGOT (AST), increased SGPT (ALT), increased creatinine, increased alkaline phosphatase, increased globulins, increased GGTP, increased/decreased/abnormal WBC, abnormal AG ratio, abnormal RBC, bilirubinemia, blood potassium increased, blood urea increased, crystal urine present, eosinophilia, hemoglobin decreased, hyperlipemia, increased/decreased electrolytes, increased/decreased cholesterol, increased glucocorticoids, increased LDH, increased/decreased/abnormal platelets, increased gastrin levels and positive fecal occult blood. Urine abnormalities such as albuminuria, glycosuria, and hematuria were also reported. Additional isolated laboratory abnormalities were reported.
In the placebo controlled studies, when SGOT (AST) and SGPT (ALT) were evaluated, 0.4% (4/978) and 0.4% (11/2677) patients, who received placebo and lansoprazole delayed-release capsules, respectively, had enzyme elevations greater than three times the upper limit of normal range at the final treatment visit. None of these patients who received lansoprazole delayed-release capsules reported jaundice at any time during the study.
In clinical trials using combination therapy with lansoprazole delayed-release capsules plus amoxicillin and clarithromycin, and lansoprazole delayed-release capsules plus amoxicillin, no increased laboratory abnormalities particular to these drug combinations were observed.
For information about laboratory value changes with antibacterial agents (amoxicillin and clarithromycin) indicated in combination with lansoprazole delayed-release capsules, refer to the ADVERSE REACTIONS section of their package inserts.
-
Drug Interactions
7.1 Drugs with pH-Dependent Absorption Kinetics
Due to its effects on gastric acid secretion, lansoprazole can reduce the absorption of drugs where gastric pH is an important determinant of their bioavailability. Like with other drugs that decrease the intragastric acidity, the absorption of drugs such as ampicillin esters, ketoconazole, atazanavir, iron salts, erlotinib, and mycophenolate mofetil (MMF) can decrease, while the absorption of drugs such as digoxin can increase during treatment with lansoprazole delayed-release capsules [see Clinical Pharmacology (12.3)].
Lansoprazole delayed-release capsules are likely to substantially decrease the systemic concentrations of the HIV protease inhibitor atazanavir, which is dependent upon the presence of gastric acid for absorption, and may result in a loss of therapeutic effect of atazanavir and the development of HIV resistance. Therefore, lansoprazole delayed-release capsules should not be co-administered with atazanavir [see Clinical Pharmacology (12.3)].
Co-administration of PPIs in healthy subjects and in transplant patients receiving MMF has been reported to reduce the exposure to the active metabolite, mycophenolic acid (MPA), possibly due to a decrease in MMF solubility at an increased gastric pH. The clinical relevance of reduced MPA exposure on organ rejection has not been established in transplant patients receiving PPIs and MMF. Use lansoprazole delayed-release capsules with caution in transplant patients receiving MMF.
7.2 Warfarin
In a study of healthy subjects, co-administration of single or multiple 60 mg doses of lansoprazole delayed-release capsules and warfarin did not affect the pharmacokinetics of warfarin nor prothrombin time (see CLINICAL PHARMACOLOGY (12.3). However, there have been reports of increased INR and prothrombin time in patients receiving PPIs and warfarin concomitantly. Increases in INR and prothrombin time may lead to abnormal bleeding and even death. Patients treated with PPIs and warfarin concomitantly may need to be monitored for increases in INR and prothrombin time. [See CLINICAL PHARMACOLOGY (12.3)]
7.3 Tacrolimus
Concomitant administration of lansoprazole and tacrolimus may increase whole blood levels of tacrolimus, especially in transplant patients who are intermediate or poor metabolizers of CYP2C19.
7.4 Theophylline
A minor increase (10%) in the clearance of theophylline was observed following the administration of lansoprazole delayed-release capsules concomitantly with theophylline. Although the magnitude of the effect on theophylline clearance is small, individual patients may require additional titration of their theophylline dosage when lansoprazole delayed-release capsules is started or stopped to ensure clinically effective blood levels. [See CLINICAL PHARMACOLOGY (12.3)]
7.5 Clopidogrel
Concomitant administration of lansoprazole and clopidogrel in healthy subjects had no clinically important effect on exposure to the active metabolite of clopidogrel or clopidogrel-induced platelet inhibition [see CLINICAL PHARMACOLOGY (12.3)]. No dose adjustment of clopidogrel is necessary when administered with an approved dose of lansoprazole delayed-release capsules.
7.6 Methotrexate
Case reports, published population pharmacokinetic studies, and retrospective analyses suggest that concomitant administration of PPIs and methotrexate (primarily at high dose; see methotrexate prescribing information) may elevate and prolong serum levels of methotrexate and/or its metabolite hydroxymethotrexate. However, no formal drug interaction studies of high dose methotrexate with PPIs have been conducted [see Warnings and Precautions (5.7)].
In a study of rheumatoid arthritis patients receiving low-dose methotrexate, lansoprazole delayed-release capsules and naproxen, no effect on pharmacokinetics of methotrexate was observed [see Clinical Pharmacology (12.3)].
7.7 Combination Therapy with Clarithromycin
Concomitant administration of clarithromycin with other drugs can lead to serious adverse reactions due to drug interactions [see Warnings and Precautions in prescribing information for clarithromycin]. Because of these drug interactions, clarithromycin is contraindicated for co-administration with certain drugs [see Contraindications in prescribing information for clarithromycin].
For information on drug interactions for amoxicillin or clarithromycin, refer to their full prescribing information, DRUG INTERACTIONS sections.
-
Overdosage
Lansoprazole delayed-release capsules are not removed from the circulation by hemodialysis. In one reported overdose, a patient consumed 600 mg of lansoprazole delayed-release capsules with no adverse reaction. Oral lansoprazole delayed-release capsules doses up to 5000 mg/kg in rats [approximately 1300 times the 30 mg human dose based on body surface area BSA)] and in mice (about 675.7 times the 30 mg human dose based on (BSA) did not produce deaths or any clinical signs.
-
Description
The active ingredient in lansoprazole delayed-release capsules USP is lansoprazole USP, a substituted benzimidazole, 2-[[[3-methyl-4-(2,2,2-trifluoroethoxy)-2-pyridyl] methyl] sulfinyl] benzimidazole, a compound that inhibits gastric acid secretion. Its empirical formula is C16H14F3N3O2S with a molecular weight of 369.37. Lansoprazole USP has the following structure:
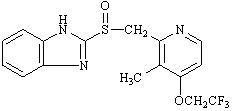
Lansoprazole USP is a white to brownish-white powder which melts with decomposition at approximately 166°C. Lansoprazole USP is freely soluble in dimethylformamideand practically insoluble in water.
Lansoprazole USP is stable when exposed to light for up to two months. The rate of degradation of the compound in aqueous solution increases with decreasing pH. The degradation half-life of the drug substance in aqueous solution at 25°C is approximately 0.5 hour at pH 5.0 and approximately 18 hours at pH 7.0.
Lansoprazole USP is supplied as delayed-release capsules.
The delayed-release capsules are available in two dosage strengths: 15 mg and 30 mg of lansoprazole USP per capsule. Each delayed-release capsule contains enteric-coated granules consisting of 15 mg or 30 mg of lansoprazole USP (active ingredient) and the following inactive ingredients: ammonium hydroxide, hydroxypropyl cellulose, low substituted hydroxypropyl cellulose, magnesium carbonate, methacrylic acid copolymer, polyethylene glycol, polysorbate 80, propylene glycol, shellac, simethicone, starch, sucrose, sugar spheres, talc, and titanium dioxide.
Components of the gelatin capsule include gelatin, iron oxide red, iron oxide yellow, FD&C Blue 2, sodium lauryl sulphate and titanium dioxide for 15 mg capsules and gelatin, iron oxide black, iron oxide red, iron oxide yellow, sodium lauryl sulphate and titanium dioxide for 30 mg capsules.
-
Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In two 24-month carcinogenicity studies, Sprague-Dawley rats were treated with oral lansoprazole doses of 5 to 150 mg/kg/day –about 1 to 40 times the exposure on a body surface (mg/m2) basis of a 50 kg person of average height [1.46 m2 body surface area (BSA)] given the recommended human dose of 30 mg/day. Lansoprazole produced dose-related gastric enterochromaffin-like (ECL) cell hyperplasia and ECL cell carcinoids in both male and female rats. It also increased the incidence of intestinal metaplasia of the gastric epithelium in both sexes. In male rats, lansoprazole produced a dose-related increase of testicular interstitial cell adenomas. The incidence of these adenomas in rats receiving doses of 15 to 150 mg/kg/day (4 to 40 times the recommended human dose based on BSA) exceeded the low background incidence (range = 1.4 to 10%) for this strain of rat.
In a 24-month carcinogenicity study, CD-1 mice were treated with oral lansoprazole doses of 15 to 600 mg/kg/day, 2 to 80 times the recommended human dose based on BSA. Lansoprazole produced a dose-related increased incidence of gastric ECL cell hyperplasia. It also produced an increased incidence of liver tumors (hepatocellular adenoma plus carcinoma). The tumor incidences in male mice treated with 300 and 600 mg/kg/day (40 to 80 times the recommended human dose based on BSA) and female mice treated with 150 to 600 mg/kg/day (20 to 80 times the recommended human dose based on BSA) exceeded the ranges of background incidences in historical controls for this strain of mice. Lansoprazole treatment produced adenoma of rete testis in male mice receiving 75 to 600 mg/kg/day (10 to 80 times the recommended human dose based on BSA).
A 26-week p53 (+/-) transgenic mouse carcinogenicity study was not positive.
Lansoprazole was positive in the Ames test and the in vitro human lymphocyte chromosomal aberration assay.Lansoprazole was not genotoxic in the ex vivo rat hepatocyte unscheduled DNA synthesis (UDS) test, the in vivo mouse micronucleus test, or the rat bone marrow cell chromosomal aberration test.
Lansoprazole at oral doses up to 150 mg/kg/day (40 times the recommended human dose based on BSA) was found to have no effect on fertility and reproductive performance of male and female rats.
13.2 Animal Toxicology and/or Pharmacology
Reproductive Toxicology Studies
Reproduction studies have been performed in pregnant rats at oral lansoprazole doses up to 150 mg/kg/day [40 times the recommended human dose (30 mg/day) based on body surface area (BSA)] and pregnant rabbits at oral lansoprazole doses up to 30 mg/kg/day (16 times the recommended human dose based on BSA) and have revealed no evidence of impaired fertility or harm to the fetus due to lansoprazole.
-
How Supplied
Lansoprazole delayed release capsules USP, 30 mg are white to pale yellow colored enteric coated pellets filled in size ‘1’ hard gelatin capsules with opaque pink colored cap and opaque black colored body, imprinted ‘RDY’ on cap and ‘399’ on body with white ink. They are supplied in bottles of 30’s, 60’s, 90's, 100’s, 500’s and unit dose package of 10 (1 x 10).
Store at 20°–25° C (68°–77° F); [See USP Controlled Room Temperature].
-
Patient Counseling Information
[See FDA-Approved Medication Guide and Patient Instructions for Use]
Patient should be informed of the following:
Advise patients to immediately report and seek care for diarrhea that does not improve. This may be a sign of Clostridium difficile associated diarrhea [see Warnings and Precautions (5.2)].
Advise patients to immediately report and seek care for any cardiovascular or neurological symptoms including palpitations, dizziness, seizures, and tetany as these may be signs of hypomagnesemia [see Warnings and Precautions (5.4)].Information for Patients
Lansoprazole is available as a capsule and is available in 15 mg and 30 mg strengths. Directions for use specific to the route and available methods of administration is presented below. [See DOSAGE AND ADMINISTRATION (2.3)]
Lansoprazole should be taken before eating.
Lansoprazole products SHOULD NOT BE CRUSHED OR CHEWED.Administration Options
1 Lansoprazole Delayed-Release Capsules
Lansoprazole delayed-release capsules should be swallowed whole.
Alternatively, for patients who have difficulty swallowing capsules, lansoprazole delayed-release capsules can be opened and administered as follows:o Open capsule.
o Sprinkle intact granules on one tablespoon of either applesauce, ENSURE pudding, cottage cheese, yogurt or strained pears.
o Swallow immediately.
Lansoprazole
o Open capsule.
o Sprinkle intact granules into a small volume of either apple juice, orange juice or tomato juice (60 mL – approximately 2 ounces).
o Mix briefly.
o Swallow immediately.
o To ensure complete delivery of the dose, the glass should be rinsed with two or more volumes of juice and the contents swallowed immediately.
Lansoprazole delayed-release capsules – Nasogastric Tube (≥16 French) Administration
For patients who have a nasogastric tube in place, lansoprazole delayed-release capsules can be administered as follows:o Open capsule.
o Mix intact granules into 40 mL of apple juice. DO NOT USE OTHER LIQUIDS.
o Inject through the nasogastric tube into the stomach.
o Flush with additional apple juice to clear the tube.
USE IN OTHER FOODS AND LIQUIDS HAS NOT BEEN STUDIED CLINICALLY AND IS THEREFORE NOT RECOMMENDED.
-
Medication Guide
MEDICATION GUIDE
Lansoprazole Delayed-Release Capsules, USP
Read this Medication Guide before you start taking lansoprazole delayed-release capsules and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
What is the most important information that I should know about lansoprazole delayed-release capsules?
Lansoprazole delayed-release capsules may help your acid-related symptoms, but you could still have serious stomach problems. Talk with your doctor.
Lansoprazole delayed-release capsules can cause serious side effects, including:
Diarrhea. Lansoprazole delayed-release capsules may increase your risk of getting severe diarrhea. This diarrhea may be caused by an infection (Clostridium difficile) in your intestines. Call your doctor right away if you have watery stool, stomach pain, and fever that does not go away.
Bone fractures. People who take multiple daily doses of proton pump inhibitor medicines for a long period of time (a year or longer) may have an increased risk of fractures of the hip, wrist or spine. You should take lansoprazole delayed-release capsules exactly as prescribed, at the lowest dose possible for your treatment and for the shortest time needed. Talk to your doctor about your risk of bone fracture if you take lansoprazole delayed-release capsules.Lansoprazole delayed-release capsules can have other serious side effects. See “What are the possible side effects of lansoprazole delayed-release capsules?”
What are lansoprazole delayed-release capsules?
Lansoprazole delayed-release capsules are prescription medicine called a proton pump inhibitor (PPI). Lansoprazole delayed-release capsules reduces the amount of acid in your stomach.
Lansoprazole delayed-release capsules are used in adults:
for 4 weeks for the healing and symptom relief of duodenal ulcers. The duodenal area is the area where food passes when it leaves the stomach.
with certain antibiotics to treat an infection called H. pylori. Sometimes H. pylori bacteria can cause duodenal ulcers. The infection needs to be treated to prevent ulcers from coming back.
for continued healing of duodenal ulcers. for up to 8 weeks to heal stomach ulcers.
for up to 8 weeks to heal stomach ulcers in some people taking pain medicines called non-steroidal anti-inflammatory drugs (NSAIDs).
for reducing the risk of stomach ulcers in some people taking NSAIDs.
for up to 8 weeks for the relief of heartburn and other symptoms of gastroesophageal reflux disease (GERD).
GERD happens when acid in your stomach backs up into the tube (esophagus) that connects your mouth to your stomach. This may cause a burning feeling in your chest or throat, sour taste or burping.
for 8 weeks to heal the acid-related damage to the lining of the esophagus (called erosive esophagitis) and to relieve symptoms. If needed, your doctor may prescribe another 8 weeks of lansoprazole delayed-release capsules.
for continued healing of erosive esophagitis.
for the long-term treatment of conditions where your stomach makes too much acid. This includes a condition called Zollinger-Ellison syndrome.Lansoprazole delayed-release capsules are used in children and adolescents (ages 1 to 17):
for up to 12 weeks to treat GERD and erosive esophagitis in children 1 to 11 years old.
for up to 8 weeks to treat GERD and erosive esophagitis in adolescents 12 to 17 years old.Lansoprazole delayed-release capsules are not effective for symptoms of GERD in children under the age of 1 year.
Who should not take lansoprazole delayed-release capsules?
Do not take lansoprazole delayed-release capsules if you are allergic to lansoprazole or any of the other ingredients in lansoprazole delayed-release capsules. See the end of this Medication Guide for a complete list of ingredients in lansoprazole delayed-release capsules.
What should I tell my doctor before taking lansoprazole delayed-release capsules?
Before you take lansoprazole delayed-release capsules, tell your doctor if you:
have been told that you have low magnesium levels in your blood.
have liver problems
have any other medical conditions
are pregnant or plan to become pregnant. It is not known if lansoprazole delayed-release capsules will harm your unborn baby.
are breastfeeding or plan to breastfeed. It is not known if lansoprazole delayed-release capsules passes into your breast milk. You and your doctor should decide if you will take lansoprazole delayed-release capsules or breastfeed. You should not do both. Talk to your doctor about the best way to feed your baby if you take lansoprazole delayed-release capsules.Tell your doctor about all the medicines you take, including prescription and non-prescription drugs, vitamins, and herbal supplements. Lansoprazole delayed-release capsules may affect how other medicines work, and other medicines may affect how lansoprazole delayed-release capsules works.
Especially tell your doctor if you take:
atazanavir (Reyataz)
erlotinib (Tarceva)
digoxin (Lanoxin)
a product that contains iron
ketoconazole (Nizoral)
warfarin (Coumadin, Jantoven)
tacrolimus (Prograf)
theophylline (Theo-24, Elixophyllin, Theochron, Theolair)
an antibiotic that contains ampicillin or clarithromycin
methotrexate
mycophenolate mofetil (Cellcept)Ask your doctor or pharmacist for a list of these medicines if you are not sure.
Know the medicines that you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take lansoprazole delayed-release capsules?
Take lansoprazole delayed-release capsules exactly as prescribed by your doctor.
Do not change your dose or stop taking lansoprazole delayed-release capsules without talking to your doctor.
You should take lansoprazole delayed-release capsules before eating.
Lansoprazole Delayed-Release Capsules:You should swallow lansoprazole delayed-release capsules whole.
Do not crush or chew lansoprazole delayed-release capsules.
If you have trouble swallowing a whole capsule, you can open the capsule and take the contents with certain foods or juices. See the “Instructions for Use” at the end of this Medication Guide for instructions on how to take lansoprazole delayed-release capsules with certain foods and juices.
See the “Instructions for Use” at the end of this Medication Guide for instructions on how to mix and give lansoprazole delayed-release capsules through a nasogastric tube.If you miss a dose, take it as soon as you remember. If it is almost time for your next dose, skip the missed dose. Just take the next dose at your regular time. Do not take 2 doses at the same time.
If you take too much lansoprazole delayed-release capsules, call your doctor right away.What are the possible side effects of lansoprazole delayed-release capsules?
Lansoprazole delayed-release capsules can cause serious side effects, including:
See “What is the most important information that I should know about lansoprazole delayed-release capsules?”
Vitamin B12 deficiency. Lansoprazole delayed-release capsules reduces the amount of acid in your stomach. Stomach acid is needed to absorb vitamin B12 properly. Talk with your doctor about the possibility of vitamin B12 deficiency if you have been on lansoprazole delayed-release capsules for a long time (more than 3 years).
Low magnesium levels in your body. This problem can be serious. Low magnesium can happen in some people who take a proton pump inhibitor medicine for at least 3 months. If low magnesium levels happen, it is usually after a year of treatment. You may or may not have symptoms of low magnesium.Tell your doctor right away if you develop any of these symptoms:
seizures
dizziness
abnormal or fast heartbeat
jitteriness
jerking movements or shaking (tremors)
muscle weakness
spasms of the hands and feet
cramps or muscle aches
spasm of the voice boxYour doctor may check the level of magnesium in your body before you start taking lansoprazole delayed-release capsules, or during treatment; if you will be taking lansoprazole delayed-release capsules for a long period of time.
The most common side effects of lansoprazole delayed-release capsules in adults and children include:
diarrhea
stomach pain
nausea
constipation
headacheOther side effects:
Serious allergic reactions. Tell your doctor if you get any of the following symptoms with lansoprazole delayed-release capsules.
rash face swelling throat tightness difficulty breathing
Your doctor may stop lansoprazole delayed-release capsules if these symptoms happen.
Tell your doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of lansoprazole delayed-release capsules. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store lansoprazole delayed-release capsules?
Store lansoprazole delayed-release capsules at room temperature between 20° to 25° C (68° to 77° F)
Keep lansoprazole delayed-release capsules and all medicines out of the reach of children.
General information about lansoprazole delayed-release capsules
Medicines are sometimes prescribed for conditions other than those listed in a Medication Guide. Do not use lansoprazole delayed-release capsules for conditions for which it was not prescribed. Do not give lansoprazole delayed-release capsules to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about lansoprazole delayed-release capsules. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about lansoprazole delayed-release capsules that is written for healthcare professionals.
For more information, call 1-888-375-3784.
What are the ingredients in lansoprazole delayed-release capsules?
Active ingredient: lansoprazole.
Inactive ingredients in lansoprazole delayed-release capsules:
Ammonium hydroxide, hydroxypropyl cellulose, low substituted hydroxypropyl cellulose, magnesium carbonate, methacrylic acid copolymer, polyethylene glycol, polysorbate 80, propylene glycol, shellac, simethicone, starch, sucrose, sugar spheres, talc, and titanium dioxide.
Components of the gelatin capsule include gelatin, iron oxide red, iron oxide yellow, FD&C Blue 2, sodium lauryl sulphate and titanium dioxide for 15 mg capsules and gelatin, iron oxide black, iron oxide red, iron oxide yellow, sodium lauryl sulphate and titanium dioxide for 30 mg capsules.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Patient Instructions for Use
Lansoprazole Delayed-Release Capsules USP
Lansoprazole Delayed-Release Capsules
Swallow lansoprazole delayed-release capsules whole. Do not crush or chew them.
You should take lansoprazole delayed-release capsules before eating.Lansoprazole Delayed-Release Capsule with certain food:
You can only use applesauce, ENSURE pudding, cottage cheese, yogurt or strained pears.
1. Open the capsule.
2. Sprinkle the granules on 1 tablespoon of either applesauce, ENSURE pudding, cottage cheese, yogurt or strained pears.
3. Swallow right away.
Lansoprazole Delayed-Release Capsule with certain juices:
You can only use apple juice, orange juice or tomato juice.
1 Open the capsule.
2 Sprinkle the granules into 60 mL (about ¼ cup) of either apple juice, orange juice or tomato juice.
3 Stir.
4 Swallow right away.
5 To make sure that the entire dose is taken, rinse the glass with 1/2 cup or more of juice to get out any leftover granules. Swallow the juice right away.
Lansoprazole Delayed-Release Capsules through a nasogastric tube (NG tube) 16 French or larger, as prescribed by your doctor:
You can only use apple juice.
1 Open the capsule and empty the granules into a syringe.
2 Do not break or crush the granules.
3 Mix with 40 mL of apple juice. Do not use other liquids.
4 Attach the syringe to the NG tube and give the medicine in the syringe through the NG tube into the stomach.
5 After giving the granules, flush the NG tube with more apple juice to clear the tube.
Lansoprazole delayed-release capsules should not be used in foods or liquids not listed above.
How should I store lansoprazole delayed-release capsules?
Store lansoprazole delayed-release capsules at room temperature between 20° to 25° C (68° to 77° F)
Keep lansoprazole delayed-release capsules and all medicines out of the reach of children.
This instruction for Use has been approved by the U.S. Food and Drug Administration.
To reorder additional Medication Guides, please contact Dr. Reddy's Customer Service at 1-866-733-3952.
All trademark names are the property of their respective owners.
Rx Only
Manufactured by:
Dr. Reddy’s Laboratories Limited
Bachupally – 500 090 INDIA
Revised: 0115
- Use in Specific Populations
- Clinical Pharmacology
- Clinical Studies
-
Dosage & Administration
Lansoprazole is available as a capsule, and is available in 15 mg and 30 mg strengths. Directions for use specific to the route and available methods of administration is presented below. Lansoprazole delayed-release capsules should be taken before eating. Lansoprazole delayed-release capsule products SHOULD NOT BE CRUSHED OR CHEWED. In the clinical trials, antacids were used concomitantly with lansoprazole delayed-release capsules.
2.1 Recommended Dose
Indication Recommended Dose Frequency
*
†
Duodenal Ulcers
Short-Term Treatment 15 mg Once daily for 4 weeks
Maintenance of Healed 15 mg Once daily
H. pylori Eradication to Reduce the Risk of Duodenal Ulcer Recurrence*
Triple Therapy:
Lansoprazole delayed-release capsules 30 mg Twice daily (q12h) for 10 or 14 days
Amoxicillin 1 gram Twice daily (q12h) for 10 or 14 days
Clarithromycin 500 mg Twice daily (q12h) for 10 or 14 days
Dual Therapy:
Lansoprazole delayed-release capsules 30 mg Three times daily (q8h) for 14 days
Amoxicillin 1 gram Three times daily (q8h) for 14 days
Benign Gastric Ulcer
Short-Term Treatment 30 mg Once daily for up to 8 weeks
NSAID-associated Gastric Ulcer
Healing 30 mg Once daily for 8 weeks†*
Risk Reduction 15 mg Once daily for up to 12 weeks†
Gastroesophageal Reflux Disease (GERD)
Short-Term Treatment of Symptomatic GERD 15 mg Once daily for up to 8 weeks
Short -Term Treatment of Erosive Esophagitis 30 mg Once daily for up to 8 weeks‡
Pediatric
(1 to 11 years of age)
Short-Term Treatment of Symptomatic GERD and Short-Term Treatment of Erosive Esophagitis
≤ 30 kg 15 mg Once daily for up to 12 weeks†§
> 30 kg 30 mg Once daily for up to 12 weeks§
(12 to 17 years of age)
Short-Term Treatment of Symptomatic GERD
Nonerosive GERD 15 mg Once daily for up to 8 weeks
Erosive Esophagitis 30 mg Once daily for up to 8 weeks
Maintenance of Healing of Erosive Esophagitis 15 mg Once daily#
Pathological Hypersecretory Conditions Including Zollinger-Ellison Syndrome 60 mg Once daily¶
* Please refer to amoxicillin and clarithromycin full prescribing information for CONTRAINDICATIONS and WARNINGS, and for information regarding dosing in elderly and renally-impaired patients.
† Controlled studies did not extend beyond indicated duration.
‡ For patients who do not heal with lansoprazole delayed-release capsules for 8 weeks (5 to 10%), it may be helpful to give an additional 8 weeks of treatment. If there is a recurrence of erosive esophagitis, an additional 8 week course of lansoprazole delayed-release capsules may be considered.
§The lansoprazole delayed-release capsules dose was increased (up to 30 mg twice daily) in some pediatric patients after 2 or more weeks of treatment if they remained symptomatic. For pediatric patients unable to swallow an intact capsule please see Administration Options.
¶ Varies with individual patient. Recommended adult starting dose is 60 mg once daily. Doses should be adjusted to individual patient needs and should continue for as long as clinically indicated. Dosages up to 90 mg twice daily have been administered. Daily dose of greater than 120 mg should be administered in divided doses. Some patients with Zollinger-Ellison Syndrome have been treated continuously with lansoprazole delayed-release capsules for more than 4 years.
# Controlled studies did not extend beyond 12 months
Patients should be instructed that if a dose is missed, it should be taken as soon as possible. However, if the next scheduled dose is due, the patient should not take the missed dose, and should be instructed to take the next dose on time. Patients should be instructed not to take 2 doses at one time to make up for a missed dose.
2.2 Special Populations
Renal impairment patients and geriatric patients do not require dosage adjustment. However, consider dose adjustment in patients with severe liver impairment. [See Use in Specific Populations (8.5, 8.6 and 8.7]
2.3 Important Administration Information
Administration Options
Lansoprazole Delayed-Release Capsules - Oral Administration
Lansoprazole delayed-release capsules should be swallowed whole.
Alternatively, for patients who have difficulty swallowing capsules, lansoprazole delayed-release capsules can be opened and administered as follows:
o Open capsule.
o Sprinkle intact granules on one tablespoon of either applesauce, ENSURE pudding, cottage cheese, yogurt or strained pears.
o Swallow immediately.
Lansoprazole Delayed-Release Capsules may also be emptied into a small volume of either apple juice, orange juice or tomato juice and administered as follows:
o Open capsule.
o Sprinkle intact granules into a small volume of either apple juice, orange juice or tomato juice (60 mL – approximately 2 ounces).
o Mix briefly.
o Swallow immediately.
o To ensure complete delivery of the dose, the glass should be rinsed with two or more volumes of juice and the contents swallowed immediately.
Lansoprazole Delayed-Release Capsules - Nasogastric Tube (≥ 16 French) Administration
For patients who have a nasogastric tube in place, lansoprazole delayed-release capsules can be administered as follows:
o Open capsule.
o Mix intact granules into 40 mL of apple juice. DO NOT USE OTHER LIQUIDS.
o Inject through the nasogastric tube into the stomach.
o Flush with additional apple juice to clear the tube.
USE IN OTHER FOODS AND LIQUIDS HAS NOT BEEN STUDIED CLINICALLY AND IS THEREFORE NOT RECOMMENDED. - Package Label. Prinicipal Display Panel
-
INGREDIENTS AND APPEARANCE
LANSOPRAZOLE
lansoprazole capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 52959-352 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LANSOPRAZOLE (UNII: 0K5C5T2QPG) (LANSOPRAZOLE - UNII:0K5C5T2QPG) LANSOPRAZOLE 30 mg Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) AMMONIA (UNII: 5138Q19F1X) HYDROXYPROPYL CELLULOSE (TYPE H) (UNII: RFW2ET671P) SHELLAC (UNII: 46N107B71O) HYDROXYPROPYL CELLULOSE, LOW SUBSTITUTED (UNII: 2165RE0K14) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STARCH, CORN (UNII: O8232NY3SJ) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) SUCROSE (UNII: C151H8M554) RAW SUGAR (UNII: 8M707QY5GH) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TALC (UNII: 7SEV7J4R1U) FERRIC OXIDE RED (UNII: 1K09F3G675) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MAGNESIUM CARBONATE (UNII: 0E53J927NA) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) GELATIN (UNII: 2G86QN327L) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) Product Characteristics Color pink (Black) Score score with uneven pieces Shape CAPSULE Size 19mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 52959-352-07 7 in 1 VIAL; Type 0: Not a Combination Product 01/09/2018 2 NDC: 52959-352-14 14 in 1 VIAL; Type 0: Not a Combination Product 01/09/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA091269 01/09/2018 Labeler - H.J. Harkins Co., Inc (147681894) Establishment Name Address ID/FEI Business Operations H.J. Harkins Co.,Inc. 147681894 manufacture(52959-352) , relabel(52959-352) , repack(52959-352)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
