PLASMANATE (plasma protein fraction- human solution
Plasmanate by
Drug Labeling and Warnings
Plasmanate by is a Other medication manufactured, distributed, or labeled by GRIFOLS USA, LLC, GRIFOLS THERAPEUTICS LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
This product has been prepared from large pools of human plasma. Each 100 mL of Plasma Protein Fraction (Human) 5%, USP—Plasmanate® contains 5 g selected plasma proteins buffered with sodium carbonate and stabilized with 0.004 M sodium caprylate and 0.004 M acetyltryptophan. The plasma proteins consist of approximately 88% normal human albumin, 12% alpha and beta globulins and not more than 1% gamma globulin as determined by electrophoresis.(1) The concentration of these proteins is such that this solution is iso-oncotic with normal human plasma and is isotonic. The approximate concentrations of the significant electrolytes in Plasmanate are: sodium 145 mEq/L, potassium 0.25 mEq/L, and chloride 100 mEq/L. Plasmanate is clear and amber colored. Plasmanate must be administered intravenously.
This product is designed to bring to the medical profession a preparation derived from human blood and similar to human plasma. Each vial of Plasmanate is sterile and heat-treated at 60°C for 10 hours against the possibility of transmitting the hepatitis viruses.
The blood group agglutinins and agglutinogens A and B are at such a low level in Plasmanate solution that its use has no effect on routine blood typing procedures. No chemical or microscopic alterations of the urine have been observed with its use.
Additionally, the manufacturing process was investigated for its capacity to decrease the infectivity of an experimental agent of transmissible spongiform encephalopathy (TSE), considered as a model for the variant Creutzfeldt-Jakob disease (vCJD) and Creutzfeldt-Jakob disease (CJD) agents. (5-8) The production steps from Pooled Plasma to Effluent IV-1 in the Plasmanate manufacturing process have been shown to decrease TSE infectivity of that experimental model agent (a total of ≥7.0 logs). These studies provide reasonable assurance that low levels of vCJD/CJD agent infectivity, if present in the starting material, would be removed.
-
CLINICAL PHARMACOLOGY
In normal human volunteers, Plasmanate has resulted in an increased blood volume which has lasted up to 48 hours.(2) Clinical experience has indicated that it is an adequate replacement for human plasma in the treatment of shock and is a suitable means of providing human proteins for their osmotic effect.
-
INDICATIONS AND USAGE
Treatment of Shock — Plasmanate is indicated in the treatment of shock due to burns, crushing injuries, abdominal emergencies, and any other cause where there is a predominant loss of plasma fluids and not red blood cells. It is also effective in the emergency treatment of shock due to hemorrhage.(3,4) Following the emergency phase of therapy, blood transfusions may be indicated depending on the severity of the blood loss.
In infants and small children, Plasmanate has been found to be very useful in the initial therapy of shock due to dehydration and infection.
-
CONTRAINDICATIONS
Plasmanate is contraindicated for use in patients on cardiopulmonary bypass. Severe hypotension has been reported in such patients when given Plasma Protein Fraction.(4)
Plasma Protein Fraction is contraindicated in patients with severe anemia, congestive heart failure, or increased blood volume.
-
WARNINGS
Plasmanate is made from human plasma. Products made from human plasma may contain infectious agents, such as viruses, and, theoretically, the Creutzfeldt-Jakob Disease (CJD) agent that can cause disease. The risk that such products will transmit an infectious agent has been reduced by screening plasma donors for prior exposure to certain viruses, by testing for the presence of certain current virus infections, and by inactivating and/or removing certain viruses. Despite these measures, such products can still potentially transmit disease. There is also the possibility that unknown infectious agents may be present in such products. Individuals who receive infusions of blood or plasma products may develop signs and/or symptoms of some viral infections, particularly hepatitis C. ALL infections thought by a physician possibly to have been transmitted by this product should be reported by the physician or other healthcare provider to Grifols Therapeutics LLC [1-800-520-2807].
The physician should discuss the risks and benefits of this product with the patient, before prescribing or administering it to the patient.
Solutions which are turbid or which have been frozen should not be used. Do not use if turbid. Do not begin administration more than 4 hours after the container has been entered. Partially used vials must be discarded. Vials which are cracked or which have been previously entered or damaged should not be used, as this may have allowed the entry of microorganisms. Plasma Protein Fraction (Human) 5%, USP—Plasmanate®contains no preservative.
-
PRECAUTIONS
General
Rapid infusion of Plasmanate (greater than 10mL/minute) has produced hypotension in patients undergoing surgery or in the preoperative or postoperative period. Blood pressure should be monitored during use and infusion slowed or ceased if sudden hypotension occurs.
Plasmanate does not provide coagulation factors and therefore does not correct coagulation disorders.
Drug Interactions
Plasmanate is compatible with whole blood, packed red cells as well as the standard carbohydrate and electrolyte solutions intended for intravenous use. It should, however, not be mixed with protein hydrolysates or solutions containing alcohol.
-
ADVERSE REACTIONS
Hypotension may occur, particularly following rapid infusion or intraarterial administration to patients on cardiopulmonary bypass. The blood pressure may normalize spontaneously after the slowing or discontinuation of the infusion. Vasopressors will also correct the hypotension.
Flushing, urticaria, back pain, nausea and headache have been occasionally reported by conscious patients.
-
DOSAGE AND ADMINISTRATION
Dosage is based almost entirely on the nature of the individual case and response to therapy. The usual minimum effective dose in adults is 250–500 mL. As with any plasma expander, the rate should be adjusted or slowed according to the clinical response and rising blood pressure.
Administration should be by vein and preferably through an area of skin at some distance from any site of infection or trauma. Plasmanate is compatible with the usual carbohydrate and electrolyte solutions.
Remove seal to expose stopper. Always swab stopper top immediately with suitable antiseptic prior to entering the vial.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Only 16 gauge needles or dispensing pins should be used with 20 mL vial sizes and larger. Needles or dispensing pins should only be inserted within the stopper area delineated by the raised ring. The stopper should be penetrated perpendicular to the plane of the stopper within the ring.
A number of factors beyond our control could reduce the efficacy of this product or even result in an ill effect following its use. These include improper storage and handling of the product after it leaves our hands, diagnosis, dosage, method of administration, and biological differences in individual patients. Because of these factors, it is important that this product be stored properly and that the directions be followed carefully during use.
- HOW SUPPLIED
- STORAGE
-
REFERENCES
-
Hink JH Jr, Hidalgo J, Seeberg VP, Johnson FF. Preparation and properties of a heat-treated human plasma protein fraction. Vox Sang. 1957;2:174–86.
-
Bertrand JJ, Feichtmeir TV, Kolomeyer N, Beatty JO, Murphy PL, Waldschmidt WD, et al. Clinical investigations with a heat-treated plasma protein fraction—Plasmanate®. Vox Sang. 1959;4:385–402.
-
Tullis JL. Albumin. 1. Background and use. 2. Guidelines for clinical use. JAMA. 1977;237:355–60; 460–3.
-
Bland JHL, Laver MB, Lowenstein E. Vasodilator effect of commercial 5% plasma protein fraction solutions. JAMA. 1973;224:1721–4.
-
Stenland CJ, Lee DC, Brown P, Petteway SR Jr, Rubenstein R. Partitioning of human and sheep forms of the pathogenic prion protein during the purification of therapeutic proteins from human plasma. Transfusion. 2002;42:1497-500.
-
Lee DC, Stenland CJ, Miller JL, Cai K, Ford EK, Gilligan KJ, et al. A direct relationship between the partitioning of the pathogenic prion protein and transmissible spongiform encephalopathy infectivity during the purification of plasma proteins. Transfusion. 2001;41:449-55.
-
Lee DC, Stenland CJ, Hartwell RC, Ford EK, Cai K, Miller JL, et al. Monitoring plasma processing steps with a sensitive Western blot assay for the detection of the prion protein. J Virol Methods. 2000;84:77-89.
-
Cai K, Miller JL, Stenland CJ, Gilligan KJ, Hartwell RC, Terry JC, et al. Solvent-dependent precipitation of prion protein. Biochim Biophys Acta. 2002;1597:28-35.
Rev. June 2018
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
3051852 -
-
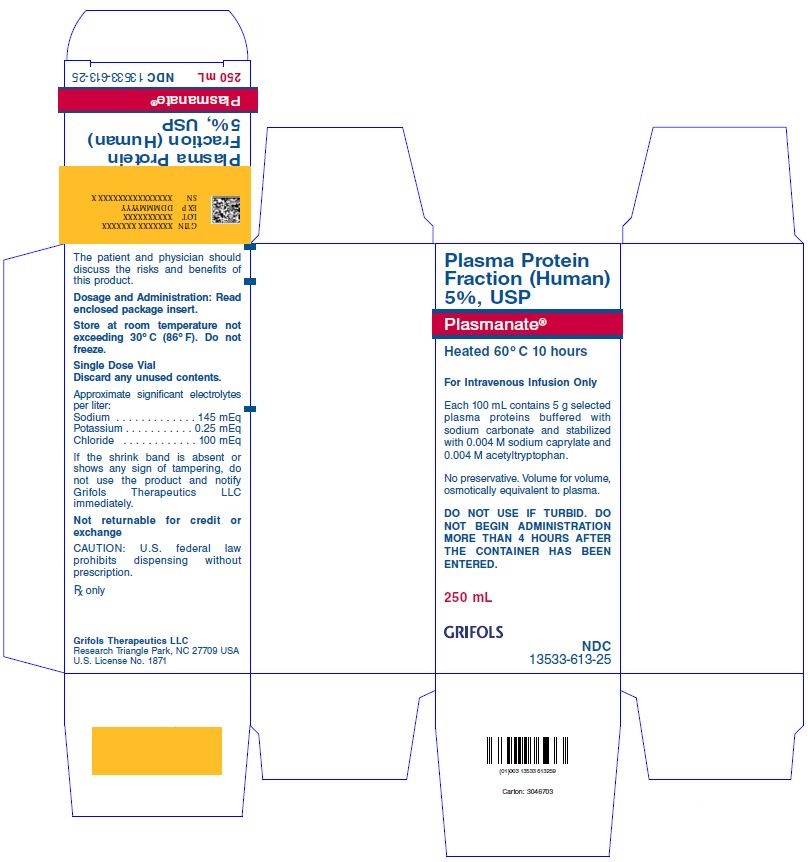
PACKAGE LABEL
Plasma Protein Fraction (Human) 5%, USP
Plasmanate®
Heated 60ºC 10 hours
For Intravenous Infusion Only
Each 100 mL contains 5 g selected plasma proteins buffered with sodium carbonate and stabilized with 0.004 M sodium caprylate and 0.004 M acetyltryptophan.
No preservative. Volume for volume, osmotically equivalent to plasma.
DO NOT USE IF TURBID. DO NOT BEGIN ADMINISTRATION MORE THAN 4 HOURS AFTER THE CONTAINER HAS BEEN ENTERED.
250 mL
GRIFOLS
NDC 13533-613-25
The patient and physician should discuss the risks and benefits of this product.
Dosage and Administration: Read enclosed package insert.
Store at room temperature not exceeding 30ºC (86ºF). Do not freeze.
Single Dose Vial
Discard any unused contents.
Approximate significant electrolytes per liter:
Sodium . . . . . . . . . . . . . .145 mEq
Potassium . . . . . . . . . . . .0.25 mEq
Chloride . . . . . . . . . . . . . .100 mEq
If the shrink band is absent or shows any sign of tampering, do not use the product and notify Grifols Therapeutics LLC immediately.
Not returnable for credit or exchange
CAUTION: U.S. federal law prohibits dispensing without prescription.
Rx only
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
Carton: 3046703
GTIN XXXXXXXXXXXXXX
LOT XXXXXXXXXX
EXP DDMMMYYYY
SN XXXXXXXXXXXXXXXX
NDC: 13533-613-26
Plasma Protein Fraction (Human) 5%, USP
Plasmanate®
Heated 60ºC 10 hours
250 mL
Grifols Therapeutics LLC
Research Triangle Park, NC 27709 USA
U.S. License No. 1871
The patient and physician should discuss the risks and benefits of this product.
DO NOT USE IF TURBID. DO NOT BEGIN ADMINISTRATION MORE THAN 4 HOURSvAFTER THE CONTAINER HAS BEEN ENTERED.
For Intravenous Infusion Only
Each 100 mL contains 5 g selected plasma proteins buffered with sodium carbonate and stabilized with 0.004 M sodium caprylate and 0.004 M acetyltryptophan.
No preservative. Volume for volume, osmotically equivalent to plasma.
Store at room temperature not exceeding 30ºC (86ºF). Do not freeze.
Dosage and Administration: Read package insert.
Single Dose Vial
Discard any unused contents.
Approximate significant electrolytes per liter:
Sodium . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 145 mEq
Potassium. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 0.25 mEq
Chloride. . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 100 mEq
Rx only
3051826
Lot
Exp.
-
INGREDIENTS AND APPEARANCE
PLASMANATE
plasma protein fraction (human) solutionProduct Information Product Type PLASMA DERIVATIVE Item Code (Source) NDC: 13533-613 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Albumin Human (UNII: ZIF514RVZR) (Albumin Human - UNII:ZIF514RVZR) Albumin Human 2.5 g in 50 mL Inactive Ingredients Ingredient Name Strength Acetyltryptophan, Dl- (UNII: 4460NBV53F) Sodium Caprylate (UNII: 9XTM81VK2B) Water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 13533-613-20 1 in 1 CARTON 1 NDC: 13533-613-21 50 mL in 1 VIAL; Type 0: Not a Combination Product 2 NDC: 13533-613-25 1 in 1 CARTON 2 NDC: 13533-613-26 250 mL in 1 VIAL; Type 0: Not a Combination Product 3 NDC: 13533-613-27 1 in 1 CARTON 3 NDC: 13533-613-28 500 mL in 1 VIAL; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA101140 10/02/1958 Labeler - GRIFOLS USA, LLC (048987452) Establishment Name Address ID/FEI Business Operations GRIFOLS THERAPEUTICS LLC 611019113 manufacture(13533-613)
Trademark Results [Plasmanate]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PLASMANATE 72228655 0819792 Live/Registered |
CUTTER LABORATORIES, INC. 1965-09-27 |
 PLASMANATE 72039861 0665653 Live/Registered |
CUTTER LABORATORIES 1957-10-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.