DIANEAL LOW CALCIUM WITH DEXTROSE- sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solution
DIANEAL LOW CALCIUM WITH DEXTROSE by
Drug Labeling and Warnings
DIANEAL LOW CALCIUM WITH DEXTROSE by is a Prescription medication manufactured, distributed, or labeled by Vantive US Healthcare LLC, Vantive Manufacturing Pte. Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Health Care Provider Letter
-
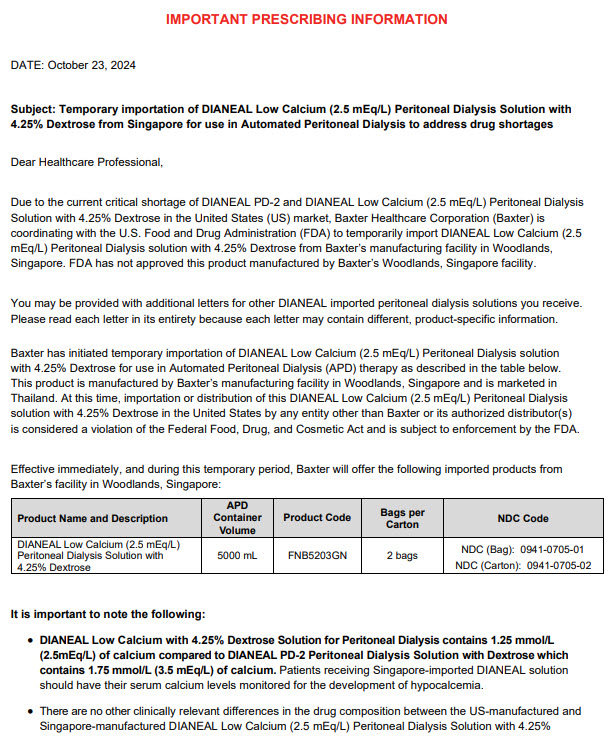
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
FNB5203
5000 mLBaxterLogo
DIANEAL Low Calcium (2.5 mEq/L) Peritoneal
Dialysis Solution with 4.25% DextroseEACH 100 mL CONTAINS 4.25 g DEXTROSE HYDROUS USP 538 mg SODIUM CHLORIDE USP
448 mg SODIUM LACTATE 18.3 mg CALCIUM CHLORIDE USP
5.08 mg MAGNESIUM CHLORIDE USP pH 5.2 (4.0 to 6.5)
mEq/L SODIUM 132 CALCIUM 2.5 MAGNESIUM 0.5 CHLORIDE 95 LACTATE 40
OSMOLARITY 483 mOsmol/L (CALC)
STERILE NONPYROGENIC STORE BELOW 30°C SEE INSERTPOTASSIUM CHLORIDE TO BE ADDED ONLY UNDER THE DIRECTION OF A PHYSICIAN
WARNING
EXTENSIVE USE OF THIS SOLUTION DURING ONE PERITONEAL DIALYSIS PROCEDURE
CAN RESULT IN SIGNIFICANT REMOVAL OF WATER FROM THE PATIENT
FOR INTRAPERITONEAL ADMINISTRATION ONLYCAUTIONS
SQUEEZE AND INSPECT INNER BAG WHICH MAINTAINS PRODUCT STERILITY
DISCARD IF LEAKS ARE FOUND DO NOT USE UNLESS SOLUTION IS CLEAR
DISCARD UNUSED PORTION KEEP OUT OF REACH OF CHILDRENBAXTER HEALTHCARE SA, SINGAPORE BRANCH AMBU-FLEXCONTAINER PL-146
2 WOODLANDS INDUSTRIAL PARK D STREET 2 SINGAPORE 737778
(AN AFFLIATE OF BAXTER HEALTHCARE CORPORATION USA)
SINGAPORE SIN6625P THAILAND 2C65/45 PHILIPPINES DR-XY20804 BRUNEI BRU12120968NP
HONG KONG HK-35010 VIETNAM VN-21179-18 MALAYSIA MAL09062144AZ
BAXTER HEALTHCARE (MALAYSIA) SDN, BHD.
B-21-3A, THE ASCENT, PARADIGM, 1, JLN SS7/26A,47301 PJ, SELANGOR
DIRECTIONS TO BE USED AS DIRECTED BY PHYSICIANJAUHI DARIPADA KANAK-KANAK
Controlled medicine/Ubat terkawalBaxter Healthcare SA, Singapore Branch
10 5 TEL 6262-7100
DIANEAL, AMBU-FLEX & PL-146are trademarks of Baxter International Inc.PVCsymbol
3A-00004
FNB5203GN
4.25% DEX
DIANEAL LOW CALCIUM PD-4
2 X 5000 ML
MAL 09062144AZBarcode
Lot:
Barcode4.25%
(2.5mEq/L)
022298Barcode
18806101552030LOT: S24A12345 EXP: 12.12.2024
-
INGREDIENTS AND APPEARANCE
DIANEAL LOW CALCIUM WITH DEXTROSE
sodium chloride, sodium lactate, calcium chloride, magnesium chloride and dextrose injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0941-0705 Route of Administration INTRAPERITONEAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROSE MONOHYDRATE (UNII: LX22YL083G) (ANHYDROUS DEXTROSE - UNII:5SL0G7R0OK) DEXTROSE MONOHYDRATE 4.25 g in 100 mL SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 538 mg in 100 mL SODIUM LACTATE (UNII: TU7HW0W0QT) (SODIUM CATION - UNII:LYR4M0NH37, LACTIC ACID, UNSPECIFIED FORM - UNII:33X04XA5AT) SODIUM LACTATE 448 mg in 100 mL CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB, CHLORIDE ION - UNII:Q32ZN48698) CALCIUM CHLORIDE 18.3 mg in 100 mL MAGNESIUM CHLORIDE (UNII: 02F3473H9O) (MAGNESIUM CATION - UNII:T6V3LHY838, CHLORIDE ION - UNII:Q32ZN48698) MAGNESIUM CHLORIDE 5.08 mg in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0941-0705-02 2 in 1 CARTON 10/28/2024 12/13/2026 1 NDC: 0941-0705-01 5000 mL in 1 BAG; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Unapproved drug for use in drug shortage 10/28/2024 12/13/2026 Labeler - Vantive US Healthcare LLC (119181963) Establishment Name Address ID/FEI Business Operations Vantive Manufacturing Pte. Ltd. 599464843 analysis(0941-0705) , label(0941-0705) , manufacture(0941-0705) , pack(0941-0705) , sterilize(0941-0705)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.