PENTACEL (diphtheria and tetanus toxoids and acellular pertussis adsorbed, inactivated poliovirus and haemophilus b conjugate- tetanus toxoid conjugate vaccine kit
PENTACEL by
Drug Labeling and Warnings
PENTACEL by is a Other medication manufactured, distributed, or labeled by Sanofi Pasteur Inc., Sanofi Pasteur Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Pentacel safely and effectively. See full prescribing information for Pentacel.
Pentacel (Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed, Inactivated Poliovirus and Haemophilus b Conjugate (Tetanus Toxoid Conjugate) Vaccine
Suspension for Intramuscular Injection
Initial U.S. Approval: 2008INDICATIONS AND USAGE
- Pentacel is a vaccine indicated for active immunization against diphtheria, tetanus, pertussis, poliomyelitis and invasive disease due to Haemophilus influenzae type b. Pentacel is approved for use as a four dose series in children 6 weeks through 4 years of age (prior to 5th birthday). (1)
DOSAGE AND ADMINISTRATION
- The four dose immunization series consists of a 0.5 mL intramuscular injection, after reconstitution, administered at 2, 4, 6 and 15-18 months of age. (2.1)
- Pentacel consists of a liquid vaccine component (DTaP-IPV component) and a lyophilized vaccine component (ActHIB vaccine). Reconstitute the ActHIB vaccine component with the DTaP-IPV component immediately before administration. (2.2)
DOSAGE FORMS AND STRENGTHS
- Suspension for injection (0.5 mL dose) supplied as a liquid vaccine component that is combined through reconstitution with a lyophilized vaccine component, both in single-dose vials. (3)
CONTRAINDICATIONS
- Severe allergic reaction (eg, anaphylaxis) after a previous dose of Pentacel, any ingredient of Pentacel, or any other diphtheria toxoid, tetanus toxoid, pertussis-containing vaccine, inactivated poliovirus vaccine or H. influenzae type b vaccine. (4.1)
- Encephalopathy within 7 days of a previous pertussis-containing vaccine with no other identifiable cause. (4.2)
- Progressive neurologic disorder until a treatment regimen has been established and the condition has stabilized. (4.3)
WARNINGS AND PRECAUTIONS
- Carefully consider benefits and risks before administering Pentacel to persons with a history of:
- - fever ≥40.5°C (≥105°F), hypotonic-hyporesponsive episode (HHE) or persistent, inconsolable crying lasting ≥3 hours within 48 hours after a previous pertussis-containing vaccine. (5.2)
- - seizures within 3 days after a previous pertussis-containing vaccine. (5.2)
- If Guillain-Barré syndrome occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the risk for Guillain-Barré syndrome may be increased following Pentacel. (5.3)
- For infants and children with a history of previous seizures, an antipyretic may be administered (in the dosage recommended in its prescribing information) at the time of vaccination with Pentacel and for the next 24 hours. (5.4)
- Apnea following intramuscular vaccination has been observed in some infants born prematurely. The decision about when to administer an intramuscular vaccine, including Pentacel, to an infant born prematurely should be based on consideration of the individual infant's medical status and the potential benefits and possible risks of vaccination. (5.7)
ADVERSE REACTIONS
- Rates of adverse reactions varied by dose number. Systemic reactions that occurred in >50% of participants following any dose included fussiness/irritability and inconsolable crying. Fever ≥38.0°C occurred in 6-16% of participants, depending on dose number. Injection site reactions that occurred in >30% of participants following any dose included tenderness and increase in arm circumference. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pharmacovigilance Department, Sanofi Pasteur Inc., Discovery Drive, Swiftwater, PA 18370 at 1-800-822-2463 (1-800-VACCINE) or VAERS at 1-800-822-7967 and http://vaers.hhs.gov.
DRUG INTERACTIONS
- Do not mix Pentacel or any of its components with any other vaccine or diluent. (7.1)
- Immunosuppressive therapies may reduce the immune response to Pentacel. (7.2)
- Urine antigen detection may not have definitive diagnostic value in suspected H. influenzae type b disease within one week following Pentacel. (7.3)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Immunization Series
2.2 Administration
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
4.2 Encephalopathy
4.3 Progressive Neurologic Disorder
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
5.2 Adverse Reactions Following Prior Pertussis Vaccination
5.3 Guillain-Barré Syndrome and Brachial Neuritis
5.4 Infants and Children with a History of Previous Seizures
5.5 Limitations of Vaccine Effectiveness
5.6 Altered Immunocompetence
5.7 Apnea in Premature Infants
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Data from Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Other Vaccines
7.2 Immunosuppressive Treatments
7.3 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.4 Pediatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Diphtheria
14.2 Tetanus
14.3 Pertussis
14.4 Poliomyelitis
14.5 Invasive Disease due to H. Influenzae Type b
14.6 Concomitantly Administered Vaccines
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Immunization Series
Pentacel is to be administered as a 4 dose series at 2, 4, 6 and 15-18 months of age. The first dose may be given as early as 6 weeks of age. Four doses of Pentacel constitute a primary immunization course against pertussis. Three doses of Pentacel constitute a primary immunization course against diphtheria, tetanus, H. influenzae type b invasive disease, and poliomyelitis; the fourth dose is a booster for diphtheria, tetanus, H. influenzae type b invasive disease, and poliomyelitis immunizations [see Clinical Studies (14.1, 14.2, 14.3, 14.4, 14.5)].
Mixed Sequences of Pentacel and DTaP Vaccine
While Pentacel and DAPTACEL (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed [DTaP], Sanofi Pasteur Limited) vaccines contain the same pertussis antigens, manufactured by the same process, Pentacel contains twice the amount of detoxified pertussis toxin (PT) and four times the amount of filamentous hemagglutinin (FHA) as DAPTACEL. Pentacel may be used to complete the first 4 doses of the 5-dose DTaP series in infants and children who have received 1 or more doses of DAPTACEL and are also scheduled to receive the other antigens of Pentacel. However, data are not available on the safety and immunogenicity of such mixed sequences of Pentacel and DAPTACEL for successive doses of the primary DTaP series. Children who have completed a 4-dose series with Pentacel should receive a fifth dose of DTaP vaccine using DAPTACEL at 4-6 years of age. (1)
Data are not available on the safety and effectiveness of using mixed sequences of Pentacel and DTaP vaccine from different manufacturers.
Mixed Sequences of Pentacel and IPV Vaccine
Pentacel may be used in infants and children who have received 1 or more doses of another licensed IPV vaccine and are scheduled to receive the antigens of Pentacel. However, data are not available on the safety and immunogenicity of Pentacel in such infants and children.
The Advisory Committee on Immunization Practices (ACIP) recommends that the final dose in the 4-dose IPV series be administered at age ≥4 years. (2) When Pentacel is administered at ages 2, 4, 6, and 15-18 months, an additional booster dose of IPV vaccine should be administered at age 4-6 years, resulting in a 5-dose IPV series. (2)
Mixed Sequences of Pentacel and Haemophilus b Conjugate Vaccine
Pentacel may be used to complete the vaccination series in infants and children previously vaccinated with one or more doses of Haemophilus b Conjugate Vaccine (either separately administered or as part of another combination vaccine), who are also scheduled to receive the other antigens of Pentacel. However, data are not available on the safety and immunogenicity of Pentacel in such infants and children. If different brands of Haemophilus b Conjugate Vaccines are administered to complete the series, three primary immunizing doses are needed, followed by a booster dose.
2.2 Administration
The package contains a vial of the DTaP-IPV component and a vial of lyophilized ActHIB vaccine component.
Before use, thoroughly but gently shake the vial of DTaP-IPV component, withdraw the entire liquid content and inject into the vial of the lyophilized ActHIB vaccine component. Gently swirl the vial now containing Pentacel until a cloudy, uniform, white to off-white (yellow tinge) suspension results.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. If these conditions exist, Pentacel should not be administered.
Withdraw and administer a single 0.5 mL dose of Pentacel intramuscularly. Pentacel should be used immediately after reconstitution. Discard unused portion. Refer to Figures 1, 2, 3, 4 and 5.
Pentacel: Instructions for Reconstitution of ActHIB Vaccine Component with DTaP-IPV Component
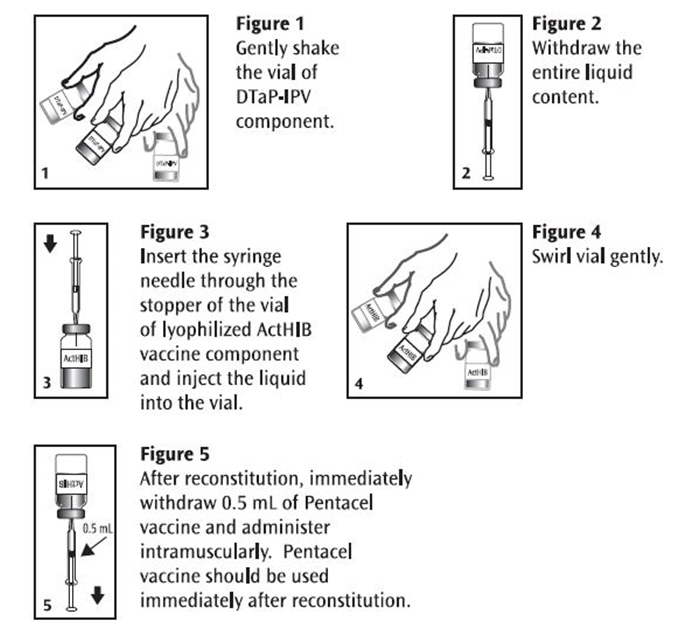
In infants younger than 1 year, the anterolateral aspect of the thigh provides the largest muscle and is the preferred site of injection. In older children, the deltoid muscle is usually large enough for injection. The vaccine should not be injected into the gluteal area or areas where there may be a major nerve trunk.
Do not administer this product intravenously or subcutaneously.
Pentacel should not be mixed in the same syringe with other parenteral products.
-
3 DOSAGE FORMS AND STRENGTHS
Pentacel is a suspension for injection (0.5 mL dose) supplied as a liquid vaccine component that is combined through reconstitution with a lyophilized vaccine component, both in single-dose vials [see Dosage and Administration (2.2) and How Supplied/Storage and Handling (16)].
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
A severe allergic reaction (eg, anaphylaxis) after a previous dose of Pentacel or any other diphtheria toxoid, tetanus toxoid, or pertussis-containing vaccine, inactivated poliovirus vaccine or H. influenzae type b vaccine, or any ingredient of this vaccine is a contraindication to administration of Pentacel [see Description (11)].
4.2 Encephalopathy
Encephalopathy (eg, coma, decreased level of consciousness, prolonged seizures) within 7 days of a previous dose of a pertussis containing vaccine that is not attributable to another identifiable cause is a contraindication to administration of any pertussis-containing vaccine, including Pentacel.
4.3 Progressive Neurologic Disorder
Progressive neurologic disorder, including infantile spasms, uncontrolled epilepsy, or progressive encephalopathy is a contraindication to administration of any pertussis-containing vaccine including Pentacel. Pertussis vaccine should not be administered to individuals with such conditions until a treatment regimen has been established and the condition has stabilized.
-
5 WARNINGS AND PRECAUTIONS
5.1 Management of Acute Allergic Reactions
Epinephrine hydrochloride solution (1:1,000) and other appropriate agents and equipment must be available for immediate use in case an anaphylactic or acute hypersensitivity reaction occurs.
5.2 Adverse Reactions Following Prior Pertussis Vaccination
If any of the following events occur within the specified period after administration of a pertussis vaccine, the decision to administer Pentacel should be based on careful consideration of potential benefits and possible risks.
- Temperature of ≥40.5°C (≥105°F) within 48 hours, not attributable to another identifiable cause.
- Collapse or shock-like state (hypotonic-hyporesponsive episode (HHE)) within 48 hours.
- Persistent, inconsolable crying lasting ≥3 hours within 48 hours.
- Seizures with or without fever within 3 days.
5.3 Guillain-Barré Syndrome and Brachial Neuritis
A review by the Institute of Medicine (IOM) found evidence for a causal relation between tetanus toxoid and both brachial neuritis and Guillain-Barré syndrome. (3) If Guillain-Barré syndrome occurred within 6 weeks of receipt of a prior vaccine containing tetanus toxoid, the risk for Guillain-Barré syndrome may be increased following Pentacel.
5.4 Infants and Children with a History of Previous Seizures
For infants or children with a history of previous seizures, an appropriate antipyretic may be administered (in the dosage recommended in its prescribing information) at the time of vaccination with a vaccine containing acellular pertussis antigens (including Pentacel) and for the following 24 hours, to reduce the possibility of post-vaccination fever.
5.6 Altered Immunocompetence
If Pentacel is administered to immunocompromised persons, including persons receiving immunosuppressive therapy, the expected immune response may not be obtained [see Drug Interactions (7.2)].
5.7 Apnea in Premature Infants
Apnea following intramuscular vaccination has been observed in some infants born prematurely. The decision about when to administer an intramuscular vaccine, including Pentacel, to an infant born prematurely should be based on consideration of the individual infant's medical status and the potential benefits and possible risks of vaccination.
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Rates of adverse reactions varied by dose number. The most frequent (>50% of participants) systemic reactions following any dose were fussiness/irritability and inconsolable crying. The most frequent (>30% of participants) injection site reactions following any dose were tenderness and increased circumference of the injected arm.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a vaccine cannot be directly compared to rates in the clinical trials of another vaccine and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to vaccine use and for approximating rates of those events.
The poliovirus component (poliovirus types 1, 2, and 3) of this formulation of Pentacel is grown in Vero cells [see Description (11)]. The clinical study data in this section were accrued with a Pentacel formulation in which the poliovirus component was grown in MRC-5 cells. The safety of Pentacel was evaluated in four clinical studies in which a total of 5,980 participants received at least one dose of Pentacel. In three of the studies, conducted in the US, a total of 4,198 participants were enrolled to receive four consecutive doses of Pentacel. In the fourth study, conducted in Canada, 1,782 participants previously vaccinated with three doses of Pentacel received a fourth dose. The vaccination schedules of Pentacel, Control vaccines, and concomitantly administered vaccines used in these studies are provided in Table 1.
Across the four studies, 50.8% of participants were female. Among participants in the three US studies, 64.5% were Caucasian, 9.2% were Black, 12.9% were Hispanic, 3.9% were Asian, and 9.5% were of other racial/ethnic groups. In the two controlled studies, the racial/ethnic distribution of participants who received Pentacel and Control vaccines was similar. In the Canadian fourth dose study, 86.0% of participants were Caucasian, 1.9% were Black, 0.8% were Hispanic, 4.3% were Asian, 2.0% were East Indian, 0.5% were Native Indian, and 4.5% were of other racial/ethnic groups.
Table 1: Clinical Safety Studies of Pentacel: Vaccination Schedules Study Pentacel Control Vaccines Concomitantly Administered Vaccines HCPDT: non-US licensed DTaP vaccine that is identical to the DTaP component of Pentacel.
POLIOVAX: US licensed Poliovirus Vaccine Inactivated, Sanofi Pasteur Limited.
IPOL: US licensed Poliovirus Vaccine Inactivated, Sanofi Pasteur SA.- * PCV7 manufactured by Wyeth Laboratories.
- † PCV7 was introduced after the study was initiated, and thus, administered concomitantly with Pentacel vaccine in a subset of participants.
- ‡ The first dose of hepatitis B vaccine (manufacturer not specified) was administered prior to study initiation, from birth to 21 days of age. Subsequent doses were with hepatitis B vaccine manufactured by Merck and Co.
- § MMR and varicella vaccines were both manufactured by Merck and Co.
- ¶ Study participants previously had received three doses of Pentacel vaccine by 8 months of age.
494-01 2, 4, 6 and 15 months HCPDT + POLIOVAX + ActHIB at 2, 4, 6, and 15 months 7-valent pneumococcal conjugate vaccine* (PCV7) at 2, 4, and 6 months in a subset of participants†
Hepatitis B vaccine at 2 and 6 months‡P3T06 2, 4, 6, and 15-16 months DAPTACEL + IPOL + ActHIB at 2, 4, and 6 months; and DAPTACEL + ActHIB at 15-16 months PCV7* at 2, 4, and 6 months
Hepatitis B vaccine at 2 and 6 months‡494-03 2, 4, 6, and 15-16 months None PCV7* at 2, 4, and 6 months in all participants; and at 15 months in a random subset of participants
Hepatitis B vaccine at 2 and 6 months (if a dose was previously administered)‡ or at 2, 4, and 6 months (if no previous dose)
Measles, mumps, rubella vaccine§ (MMR) and varicella§ vaccine at 12 or 15 months in random subsets of participants5A9908 15-18 months¶ None None Solicited Adverse Reactions
The incidence and severity of selected solicited injection site and systemic adverse reactions that occurred within 3 days following each dose of Pentacel or Control vaccines in Study P3T06 is shown in Table 2. Information on these reactions was recorded daily by parents or guardians on diary cards. In Table 2, injection site reactions are reported for the Pentacel and DAPTACEL injection sites.
Table 2: Number (Percentage) of Children with Selected Solicited Adverse Reactions by Severity Occurring within 0-3 days of Pentacel or Control Vaccines in Study P3T06 - * Any: Mild, Moderate or Severe; Mild: subject whimpers when site is touched; Moderate: subject cries when site is touched; Severe: subject cries when leg or arm is moved.
- † Fever is based upon actual temperatures recorded with no adjustments to the measurement route.
- ‡ Following Doses 1-3 combined, the proportion of temperature measurements that were taken by axillary, rectal or other routes, or not recorded were 46.0%, 53.0%, 1.0%, and 0% respectively, for Pentacel vaccine and 44.8%, 54.0%, 1.0%, and 0.1%, respectively, for DAPTACEL + IPOL + ActHIB. Following Dose 4, the proportion of temperature measurements that were taken by axillary, rectal or other routes, or not recorded were 62.7%, 34.4%, 2.4% and 0.5%, respectively, for Pentacel vaccine, and 61.1%, 36.6%, 1.7% and 0.5%, respectively, for DAPTACEL + ActHIB.
- § Moderate: interferes with or limits usual daily activity; Severe: disabling, not interested in usual daily activity.
Injection Site Reactions Pentacel DAPTACEL Dose 1
N = 465-467
%Dose 2
N = 451
%Dose 3
N = 438-440
%Dose 4
N = 387-396
%Dose 1
N = 1,400-1,404
%Dose 2
N = 1,358-1,359
%Dose 3
N = 1,311-1,312
%Dose 4
N = 376-380
%Redness >5 mm 7.1 8.4 8.7 17.3 6.2 7.1 9.6 16.4 >25 mm 2.8 1.8 1.8 9.2 1.0 0.6 1.9 7.9 >50 mm 0.6 0.2 0.0 2.3 0.4 0.1 0.0 2.4 Swelling >5 mm 7.5 7.3 5.0 9.7 4.0 4.0 6.5 10.3 >25 mm 3.0 2.0 1.6 3.8 1.6 0.7 1.1 4.0 >50 mm 0.9 0.0 0.0 0.8 0.4 0.1 0.1 1.3 Tenderness* Any 47.5 39.2 42.7 56.1 48.8 38.2 40.9 51.1 Moderate or Severe 19.6 10.6 11.6 16.7 20.7 12.2 12.3 15.8 Severe 5.4 1.6 1.4 3.3 4.1 2.3 1.7 2.4 Increase in Arm Circumference >5 mm 33.6 30.6 >20 mm – – – 4.7 – – – 6.9 >40 mm 0.5 0.8 Systemic Reactions Pentacel DAPTACEL + IPOL + ActHIB DAPTACEL + ActHIB Dose 1
N = 466-467
%Dose 2
N = 451-452
%Dose 3
N = 435-440
%Dose 4
N = 389-398
%Dose 1
N = 1,390-1,406
%Dose 2
N = 1,346-1,360
%Dose 3
N = 1,301-1,312
%Dose 4
N = 379-381
%Fever†‡ ≥38.0°C 5.8 10.9 16.3 13.4 9.3 16.1 15.8 8.7 >38.5°C 1.3 2.4 4.4 5.1 1.6 4.3 5.1 3.2 >39.5°C 0.4 0.0 0.7 0.3 0.1 0.4 0.3 0.8 Decreased Activity/Lethargy§ Any 45.8 32.7 32.5 24.1 51.1 37.4 33.2 24.1 Moderate or Severe 22.9 12.4 12.7 9.8 24.3 15.8 12.7 9.2 Severe 2.1 0.7 0.2 2.5 1.2 1.4 0.6 0.3 Inconsolable Crying Any 59.3 49.8 47.3 35.9 58.5 51.4 47.9 36.2 ≥1 hour 19.7 10.6 13.6 11.8 16.4 16.0 12.2 10.5 >3 hours 1.9 0.9 1.1 2.3 2.2 3.4 1.4 1.8 Fussiness/Irritability Any 76.9 71.2 68.0 53.5 75.8 70.7 67.1 53.8 ≥1 hour 34.5 27.0 26.4 23.6 33.3 30.5 26.2 19.4 >3 hours 4.3 4.0 5.0 5.3 5.6 5.5 4.3 4.5 Hypotonic Hyporesponsive Episodes
In Study P3T06, the diary cards included questions pertaining to HHEs. In Studies 494-01, 494-03, and 5A9908, a question about the occurrence of fainting or change in mental status was asked during post-vaccination phone calls. Across these 4 studies, no HHEs, as defined in a report of a US Public Health Service workshop (4) were reported among participants who received Pentacel (N = 5,979), separately administered HCPDT + POLIOVAX + ActHIB (N = 1,032) or separately administered DAPTACEL + IPOL + ActHIB (N = 1,455). Hypotonia not fulfilling HHE criteria within 7 days following vaccination was reported in 4 participants after the administration of Pentacel (1 on the same day as the 1st dose; 3 on the same day as the 3rd dose) and in 1 participant after the administration of DAPTACEL + IPOL + ActHIB (4 days following the 1st dose).
Seizures
Across Studies 494-01, 494-03, 5A9908 and P3T06, a total of 8 participants experienced a seizure within 7 days following either Pentacel (4 participants; N = 4,197 for at least one of Doses 1-3; N = 5,033 for Dose 4), separately administered HCPDT + POLIOVAX + ActHIB (3 participants; N = 1,032 for at least one of Doses 1-3, N = 739 for Dose 4), separately administered DAPTACEL + IPOL + ActHIB (1 participant; N = 1,455 for at least one of Doses 1-3), or separately administered DAPTACEL + ActHIB (0 participants; N = 418 for Dose 4). Among the four participants who experienced a seizure within 7 days following Pentacel, one participant in Study 494-01 had an afebrile seizure 6 days after the first dose, one participant in Study 494-01 had a possible seizure the same day as the third dose, and two participants in Study 5A9908 had a febrile seizure 2 and 4 days, respectively, after the fourth dose. Among the four participants who experienced a seizure within 7 days following Control vaccines, one participant had an afebrile seizure the same day as the first dose of DAPTACEL + IPOL + ActHIB, one participant had an afebrile seizure the same day as the second dose of HCPDT + POLIOVAX + ActHIB, and two participants had a febrile seizure 6 and 7 days, respectively, after the fourth dose of HCPDT + POLIOVAX + ActHIB.
Serious Adverse Events
In Study P3T06, within 30 days following any of Doses 1-3 of Pentacel or Control vaccines, 19 of 484 (3.9%) participants who received Pentacel and 50 of 1,455 (3.4%) participants who received DAPTACEL + IPOL + ActHIB experienced a serious adverse event. Within 30 days following Dose 4 of Pentacel or Control vaccines, 5 of 431 (1.2%) participants who received Pentacel and 4 of 418 (1.0%) participants who received DAPTACEL + ActHIB experienced a serious adverse event. In Study 494-01, within 30 days following any of Doses 1-3 of Pentacel or Control vaccines, 23 of 2,506 (0.9%) participants who received Pentacel and 11 of 1,032 (1.1%) participants who received HCPDT + POLIOVAX + ActHIB experienced a serious adverse event. Within 30 days following Dose 4 of Pentacel or Control vaccines, 6 of 1,862 (0.3%) participants who received Pentacel and 2 of 739 (0.3%) participants who received HCPDT + POLIOVAX + ActHIB experienced a serious adverse event.
Across Studies 494-01, 494-03 and P3T06, within 30 days following any of Doses 1-3 of Pentacel or Control vaccines, overall, the most frequently reported serious adverse events were bronchiolitis, dehydration, pneumonia and gastroenteritis. Across Studies 494-01, 494-03, 5A9908 and P3T06, within 30 days following Dose 4 of Pentacel or Control vaccines, overall, the most frequently reported serious adverse events were dehydration, gastroenteritis, asthma, and pneumonia.
Across Studies 494-01, 494-03, 5A9908 and P3T06, two cases of encephalopathy were reported, both in participants who had received Pentacel (N = 5,979). One case occurred 30 days post-vaccination and was secondary to cardiac arrest following cardiac surgery. One infant who had onset of neurologic symptoms 8 days post-vaccination was subsequently found to have structural cerebral abnormalities and was diagnosed with congenital encephalopathy.
A total of 5 deaths occurred during Studies 494-01, 494-03, 5A9908 and P3T06: 4 in children who had received Pentacel (N = 5,979) and one in a participant who had received DAPTACEL + IPOL + ActHIB (N = 1,455). There were no deaths reported in children who received HCPDT + POLIOVAX + ActHIB (N = 1,032). Causes of death among children who received Pentacel were asphyxia due to suffocation, head trauma, Sudden Infant Death syndrome, and neuroblastoma (8, 23, 52 and 256 days post-vaccination, respectively). One participant with ependymoma died secondary to aspiration 222 days following DAPTACEL + IPOL + ActHIB.
6.2 Data from Postmarketing Experience
The following additional adverse events have been spontaneously reported during the post- marketing use of Pentacel worldwide, since 1997. Between 1997 and 2007, Pentacel was primarily used in Canada. Because these events are reported voluntarily from a population of uncertain size, it may not be possible to reliably estimate their frequency or establish a causal relationship to vaccine exposure.
The following adverse events were included based on one or more of the following factors: severity, frequency of reporting, or strength of evidence for a causal relationship to Pentacel.
-
Cardiac disorders
Cyanosis -
Gastrointestinal disorders
Vomiting, diarrhea -
General disorders and administration site conditions
Injection site reactions (including inflammation, mass, abscess and sterile abscess), extensive swelling of the injected limb (including swelling that involved adjacent joints), vaccination failure/therapeutic response decreased (invasive H. influenzae type b disease) -
Immune system disorders
Anaphylaxis/anaphylactic reaction, hypersensitivity (such as rash and urticaria) -
Infections and infestations
Meningitis, rhinitis, viral infection -
Metabolism and nutrition disorders
Decreased appetite -
Nervous system disorders
Somnolence, HHE, depressed level of consciousness -
Psychiatric disorders
Screaming -
Respiratory, thoracic and mediastinal disorders
Apnea, cough -
Skin and subcutaneous tissue disorders
Erythema, skin discoloration -
Vascular disorders
Pallor
-
7 DRUG INTERACTIONS
7.1 Concomitant Administration with Other Vaccines
In clinical trials, Pentacel was administered concomitantly with one or more of the following US licensed vaccines: hepatitis B vaccine, 7-valent pneumococcal conjugate vaccine, MMR and varicella vaccines [see Adverse Reactions (6) and Clinical Studies (14)]. When Pentacel is given at the same time as another injectable vaccine(s), the vaccine(s) should be administered with different syringes and at different injection sites.
7.2 Immunosuppressive Treatments
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs and corticosteroids (used in greater than physiologic doses), may reduce the immune response to Pentacel [see Warnings and Precautions (5.6)].
7.3 Drug/Laboratory Test Interactions
Antigenuria has been detected in some instances following receipt of ActHIB. Urine antigen detection may not have definite diagnostic value in suspected H. influenzae type b disease within one week following receipt of Pentacel. (5)
-
8 USE IN SPECIFIC POPULATIONS
8.4 Pediatric Use
The safety and effectiveness of Pentacel was established in the age group 6 weeks through 18 months on the basis of clinical studies [see Clinical Trials Experience (6.1) and Clinical Studies (14)]. The safety and effectiveness of Pentacel in the age group 19 months through 4 years is supported by evidence in children 6 weeks through 18 months. The safety and effectiveness of Pentacel in infants less than 6 weeks of age and in children 5 to 16 years of age have not been established.
-
11 DESCRIPTION
Pentacel consists of a Diphtheria and Tetanus Toxoids and Acellular Pertussis Adsorbed and Inactivated Poliovirus (DTaP-IPV) component and an ActHIB® component combined through reconstitution for intramuscular injection. ActHIB (Haemophilus b Conjugate Vaccine [Tetanus Toxoid Conjugate]), consists of H. influenzae type b capsular polysaccharide (polyribosyl-ribitol-phosphate [PRP]) covalently bound to tetanus toxoid (PRP-T). The DTaP-IPV component is supplied as a sterile liquid used to reconstitute the lyophilized ActHIB component to form Pentacel. Pentacel is a uniform, cloudy, white to off-white (yellow tinge) suspension.
Each 0.5 mL dose contains 15 Lf diphtheria toxoid, 5 Lf tetanus toxoid, acellular pertussis antigens [20 mcg detoxified pertussis toxin (PT), 20 mcg filamentous hemagglutinin (FHA), 3 mcg pertactin (PRN), 5 mcg fimbriae types 2 and 3 (FIM)], inactivated polioviruses [29 D-antigen units (DU) Type 1 (Mahoney), 7 DU Type 2 (MEF-1), 26 DU Type 3 (Saukett)] and 10 mcg PRP of H. influenzae type b covalently bound to 24 mcg of tetanus toxoid (PRP-T).
Other ingredients per 0.5 mL dose include 1.5 mg aluminum phosphate (0.33 mg aluminum) as the adjuvant, <8.1 mcg polysorbate 80, 3.3 mg (0.6% v/v) 2-phenoxyethanol (not as a preservative), 42.5 mg sucrose, 2 mcg to 7 mcg residual formaldehyde, <50 ng residual glutaraldehyde, ≤10 ng residual bovine serum albumin, <0.0001 pg streptomycin sulphate, <0.01 pg of neomycin and <0.000001 pg polymyxin B sulphate.
Corynebacterium diphtheriae is grown in modified Mueller's growth medium. (6) After purification by ammonium sulfate fractionation, the diphtheria toxin is detoxified with formaldehyde and diafiltered.
Clostridium tetani is grown in modified Mueller-Miller casamino acid medium without beef heart infusion. (7) Tetanus toxin is detoxified with formaldehyde and purified by ammonium sulfate fractionation and diafiltration. Diphtheria and tetanus toxoids are individually adsorbed onto aluminum phosphate.
The acellular pertussis vaccine antigens are produced from Bordetella pertussis cultures grown in Stainer-Scholte medium (8) modified by the addition of casamino acids and dimethyl-beta-cyclodextrin. PT, FHA and PRN are isolated separately from the supernatant culture medium. FIM are extracted and copurified from the bacterial cells. The pertussis antigens are purified by sequential filtration, salt-precipitation, ultrafiltration and chromatography. PT is detoxified with glutaraldehyde. FHA is treated with formaldehyde and the residual aldehydes are removed by ultrafiltration. The individual antigens are adsorbed separately onto aluminum phosphate.
The Type 1, Type 2, and Type 3 polioviruses are individually grown in Vero cells (a continuous line of monkey kidney cells). Prior to viral propagation, the cells are grown in Iscove's medium, supplemented with calf serum. For viral propagation, the culture medium is replaced by M199 medium without calf serum. The viral harvests are concentrated and purified, then inactivated with formaldehyde to produce monovalent suspensions of each serotype. Specified quantities of monovalent suspensions of each serotype are mixed to produce the trivalent poliovirus concentrate.
The adsorbed diphtheria, tetanus and acellular pertussis antigens are combined with aluminum phosphate (as adjuvant), 2-phenoxyethanol (not as a preservative) and water for injection, into an intermediate concentrate. The trivalent poliovirus concentrate is added and the DTaP-IPV component is diluted to its final concentration. The DTaP-IPV component does not contain a preservative.
Both diphtheria and tetanus toxoids induce at least 2 neutralizing units per mL in the guinea pig potency test. The potency of the acellular pertussis antigens is evaluated by the antibody response of immunized mice to detoxified PT, FHA, PRN and FIM as measured by enzyme-linked immunosorbent assay (ELISA). The potency of inactivated poliovirus antigens is determined by measuring antibody-mediated neutralization of poliovirus in sera from immunized rats.
PRP, a high molecular weight polymer, is prepared from the Haemophilus influenzae type b strain 1482 grown in a semi-synthetic medium. (9) The tetanus toxoid for conjugation to PRP is prepared by ammonium sulfate purification, and formalin inactivation of the toxin from cultures of Clostridium tetani (Harvard strain) grown in a modified Mueller and Miller medium. (10) The toxoid is filter sterilized prior to the conjugation process. The ActHIB component does not contain a preservative. Potency of the ActHIB component is specified on each lot by limits on the content of PRP polysaccharide and protein per dose and the proportion of polysaccharide and protein that is characterized as high molecular weight conjugate.
The vial stoppers for the DTaP-IPV and ActHIB components of Pentacel are not made with natural rubber latex.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Diphtheria
Diphtheria is an acute toxin-mediated disease caused by toxigenic strains of C. diphtheriae. Protection against disease is due to the development of neutralizing antibodies to diphtheria toxin. A serum diphtheria antitoxin level of 0.01 IU/mL is the lowest level giving some degree of protection. Antitoxin levels of at least 0.1 IU/mL are generally regarded as protective. (11) Levels of 1.0 IU/mL have been associated with long-term protection. (12)
Tetanus
Tetanus is an acute disease caused by an extremely potent neurotoxin produced by C. tetani. Protection against disease is due to the development of neutralizing antibodies to tetanus toxin. A serum tetanus antitoxin level of at least 0.01 IU/mL, measured by neutralization assay is considered the minimum protective level. (11) (13) A tetanus antitoxoid level ≥0.1 IU/mL as measured by the ELISA used in clinical studies of Pentacel is considered protective.
Pertussis
Pertussis (whooping cough) is a respiratory disease caused by B. pertussis. This Gram-negative coccobacillus produces a variety of biologically active components, though their role in either the pathogenesis of, or immunity to, pertussis has not been clearly defined.
Poliomyelitis
Polioviruses, of which there are three serotypes (Types 1, 2, and 3) are enteroviruses. The presence of poliovirus type-specific neutralizing antibodies has been correlated with protection against poliomyelitis. (14)
Invasive Disease Due to H. influenzae Type b
H. influenzae type b can cause invasive disease such as meningitis and sepsis. Anti-PRP antibody has been shown to correlate with protection against invasive disease due to H. influenzae type b.
Based on data from passive antibody studies (15) and an efficacy study with H. influenzae type b polysaccharide vaccine in Finland, (16) a post-vaccination anti-PRP level of 0.15 mcg/mL has been accepted as a minimal protective level. Data from an efficacy study with H. influenzae type b polysaccharide vaccine in Finland indicate that a level >1.0 mcg/mL 3 weeks after vaccination predicts protection through a subsequent one-year period. (17) (18) These levels have been used to evaluate the effectiveness of Haemophilus b Conjugate Vaccines, including the ActHIB component of Pentacel.
- 13 NONCLINICAL TOXICOLOGY
-
14 CLINICAL STUDIES
The efficacy of Pentacel is based on the immunogenicity of the individual antigens compared to separately administered vaccines. The poliovirus component (poliovirus types 1, 2 and 3) of this formulation of Pentacel is grown in Vero cells [see Description (11)]. The clinical study data in this section were accrued with a Pentacel formulation in which the poliovirus component was grown in MRC-5 cells. The poliovirus component of the two Pentacel formulations are analytically comparable. Serological correlates of protection exist for diphtheria, tetanus, poliomyelitis, and invasive disease due to H. influenzae type b [see Clinical Pharmacology (12.1)]. The efficacy against pertussis, for which there is no well established serological correlate of protection, was based, in part, on a comparison of pertussis immune responses following Pentacel in US children to responses following DAPTACEL (Diphtheria and Tetanus Toxoids and Acellular Pertussis Vaccine Adsorbed (DTaP) manufactured by Sanofi Pasteur Limited) in an efficacy study conducted in Sweden (Sweden I Efficacy Trial). While Pentacel and DAPTACEL contain the same pertussis antigens, manufactured by the same process, Pentacel contains twice as much detoxified PT and four times as much FHA as DAPTACEL.
Immune responses to Pentacel were evaluated in four US studies: Studies 494-01, P3T06, 494-03, and M5A10. The vaccination schedules of Pentacel, Control vaccines, and concomitantly administered vaccines used in Studies 494-01, P3T06, and 494-03 are provided in Table 1 [see Clinical Trials Experience (6.1)]. In Study M5A10, participants were randomized to receive Pentacel or separately administered DAPTACEL, IPOL, and ActHIB at 2, 4, and 6 months of age. 7-valent pneumococcal conjugate (PCV7, Wyeth Pharmaceuticals Inc.) at 2, 4, and 6 months of age, and Hepatitis B vaccine (Merck and Co. or GlaxoSmithKline Biologicals) at 2 and 6 months of age, were administered concomitantly with Pentacel or Control vaccines.
14.1 Diphtheria
The proportions of participants achieving diphtheria antitoxin seroprotective levels one month following three and four doses of Pentacel or DAPTACEL in Study P3T06 are provided in Table 3.
14.2 Tetanus
The proportions of participants achieving tetanus antitoxoid seroprotective levels one month following three and four doses of Pentacel or DAPTACEL in Study P3T06 are provided in Table 3.
Table 3: Study P3T06 Diphtheria Antitoxin and Tetanus Antitoxoid Responses One Month Following Dose 3 and Dose 4 of Pentacel or DAPTACEL + IPOL + ActHIB in US Children Vaccinated at 2, 4, 6, and 15-16 Months of Age Pentacel DAPTACEL + IPOL
+ ActHIBPer Protocol Immunogenicity population.
- * Seroprotection rate following Pentacel vaccine is not inferior to DAPTACEL vaccine (upper limit of 90% CI of the difference DAPTACEL – Pentacel is <10%).
- † Non-inferiority criteria were not pre-specified.
- ‡ With the ELISA used in this study, a tetanus antitoxoid level of 1.0 IU/mL is 10 times the protective level.
Post-Dose 3 N = 331-345 N = 1,037-1,099 Diphtheria Antitoxin
% ≥0.01 IU/mL*
% ≥0.10 IU/mL†
Tetanus Antitoxoid
% ≥0.10 IU/mL†
100.0%
98.8%
99.7%
100.0%
98.5%
100.0%Post-Dose 4 N = 341-352 N = 328-334 Diphtheria Antitoxin
% ≥0.10 IU/mL*
% ≥1.0 IU/mL†
Tetanus Antitoxoid
% ≥0.10 IU/mL*
% ≥1.0 IU/mL†‡
100.0%
96.5%
100.0%
92.9%
100.0%
95.7%
100.0%
99.4%14.3 Pertussis
In a clinical pertussis vaccine efficacy study conducted in Sweden during 1992-1995 (Sweden I Efficacy Trial), 2,587 infants received DAPTACEL and 2,574 infants received a non-US licensed DT vaccine as placebo at 2, 4, and 6 months of age. (1) The mean length of follow-up was 2 years after the third dose of vaccine. The protective efficacy of DAPTACEL against pertussis after 3 doses of vaccine using the World Health Organization (WHO) case definition (≥21 consecutive days of paroxysmal cough with culture or serologic confirmation or epidemiologic link to a confirmed case) was 84.9% (95% confidence interval [CI] 80.1%, 88.6%). The protective efficacy of DAPTACEL against mild pertussis (≥1 day of cough with laboratory confirmation) was 77.9% (95% CI 72.6%, 82.2%). Protection against pertussis by DAPTACEL was sustained for the 2-year follow-up period.
Based on comparisons of the immune responses to DAPTACEL in US infants (Post-Dose 3) and Canadian children (Post-Dose 4) relative to infants who participated in the Sweden I Efficacy Trial, it was concluded that 4 doses of DAPTACEL were needed for primary immunization against pertussis in US children. (1)
In a serology bridging analysis, immune responses to FHA, PRN and FIM in a subset of infants who received three doses of DAPTACEL in the Sweden I Efficacy Trial were compared to the Post-Dose 3 and Post-Dose 4 responses in a subset of US children from Study 494-01 who received Pentacel (Table 4). Available stored sera from infants who received DAPTACEL in the Sweden I Efficacy Trial and sera from children who received PCV7 concomitantly with the first three doses of Pentacel in Study 494-01 (Table 1) were assayed in parallel. Data on levels of antibody to PT using an adequately specific assay were not available for this serology bridging analysis.
Geometric mean antibody concentrations (GMCs) and seroconversion rates for antibodies to FHA, PRN and FIM one month following Dose 3 of DAPTACEL in the subset of infants from the Sweden I Efficacy Trial and one month following Dose 3 and Dose 4 of Pentacel in a subset of infants from US Study 494-01 are presented in Table 4. Seroconversion was defined as 4-fold rise in antibody level (Post-Dose 3/Pre-Dose 1 or Post-Dose 4/Pre-Dose 1). For anti-FHA and anti-FIM, the non-inferiority criteria were met for seroconversion rates, and for anti-FHA, anti-PRN, and anti-FIM, the non-inferiority criteria were met for GMCs, following Dose 4 of Pentacel relative to Dose 3 of DAPTACEL. The non-inferiority criterion for anti-PRN seroconversion following Dose 4 of Pentacel relative to Dose 3 of DAPTACEL was not met [upper limit of 95% CI for difference in rate (DAPTACEL minus Pentacel) = 13.24%]. Whether the lower anti-PRN seroconversion rate following Dose 4 of Pentacel in US children relative to Dose 3 of DAPTACEL in Swedish infants correlates with diminished efficacy of Pentacel against pertussis is unknown.
Table 4: FHA, PRN and FIM Antibody Responses One Month Following Dose 3 of DAPTACEL in a Subset of Infants Vaccinated at 2, 4, and 6 Months of Age in the Sweden I Efficacy Trial and One Month Following Dose 3 and Dose 4 of Pentacel in a Subset of Infants Vaccinated at 2, 4, 6, and 15-16 Months of Age in US Study 494-01 Post-Dose 3 DAPTACEL
Sweden I Efficacy Trial
N = 80Post-Dose 3
Pentacel*
US Study 494-01
N = 730-995Post-Dose 4
Pentacel†
US Study 494-01
N = 507-554Analyzed sera were from subsets of the Per Protocol Immunogenicity populations in each study.
Data on anti-PT levels using an adequately specific assay were not available.- * Non-inferiority criteria were not pre-specified for the comparisons of immune responses to Pentacel vaccine Post-Dose 3 vs. DAPTACEL vaccine Post-Dose 3.
- † Pre-specified non-inferiority analyses compared immune responses to Pentacel vaccine Post-Dose 4 vs. DAPTACEL vaccine Post-Dose 3.
- ‡ Fold rise was calculated as Post-Dose 3/Pre-Dose 1 antibody level or Post-Dose 4/Pre-Dose 1 antibody level.
- § Percent achieving 4-fold rise or GMC Post-Dose 4 Pentacel vaccine is not inferior to Post-Dose 3 DAPTACEL vaccine [upper limit of 95% CI for difference in rates (DAPTACEL minus Pentacel) <10% and upper limit of 90% CI for GMC ratio (DAPTACEL/Pentacel) <1.5].
- ¶ Non-inferiority criterion is not met for percent achieving 4-fold rise in anti-PRN Post-Dose 4 Pentacel vaccine relative to Post-Dose 3 DAPTACEL vaccine [upper limit of 95% CI for difference in rates (DAPTACEL minus Pentacel) = 13.24%, exceeds the non-inferiority criterion of <10%].
Anti-FHA
% achieving 4-fold rise‡
GMC (EU/mL)
68.8
40.70
79.8
71.46
91.7§
129.85§Anti-PRN
% achieving 4-fold rise‡
GMC (EU/mL)
98.8
111.26
74.4
38.11
89.2¶
90.82§Anti-FIM
% achieving 4-fold rise‡
GMC (EU/mL)
86.3
339.31
86.5
265.02
91.5§
506.57§In a separate study, Study P3T06, US infants were randomized to receive either Pentacel or DAPTACEL + IPOL + ActHIB at 2, 4, 6, and 15-16 months of age (Table 1). The pertussis immune responses (GMCs and seroconversion rates) one month following the third and fourth doses were compared between the two groups (Table 5). Seroconversion was defined as a 4-fold rise in antibody level (Post-Dose 3/Pre-Dose 1 or Post-Dose 4/Pre-Dose 1). Data on anti-PT responses obtained from an adequately specific assay were available on only a non-random subset of study participants. The subset of study participants was representative of all study participants with regard to Pre-Dose 1, Post-Dose 3 and Post-Dose 4 GMCs of antibodies to FHA, PRN and FIM. For each of the pertussis antigens, non-inferiority criteria were met for seroconversion rates and GMCs following Dose 3 of Pentacel relative to Dose 3 of DAPTACEL. Following Dose 4 of Pentacel relative to Dose 4 of DAPTACEL, non-inferiority criteria were met for all comparisons except for anti-PRN GMCs [upper limit of 90% CI for ratio of GMCs (DAPTACEL/Pentacel) = 2.25]. Whether the lower anti-PRN GMC following Dose 4 of Pentacel relative to Dose 4 of DAPTACEL in US children correlates with diminished efficacy of Pentacel against pertussis is unknown.
Table 5: Pertussis Antibody Responses One Month Following Doses 3 and 4 of Pentacel or DAPTACEL + IPOL + ActHIB in US Infants Vaccinated at 2, 4, 6, and 15-16 Months of Age in Study P3T06 Post-Dose 3
PentacelPost-Dose 3
DAPTACEL + IPOL + ActHIBPost-Dose 4
PentacelPost-Dose 4
DAPTACEL + ActHIBPer Protocol Immunogenicity population for anti-FHA, anti-PRN, and anti-FIM.
Non-random subset of per Protocol Immunogenicity population for anti-PT. See text for further information on the subset evaluated.- * Fold rise was calculated as Post-Dose 3/Pre-Dose 1 antibody level or Post-Dose 4/Pre-Dose 1 antibody level.
- † Percent achieving 4-fold rise or GMC Post-Dose 3 Pentacel vaccine not inferior to Post-Dose 3 DAPTACEL vaccine [upper limit of 95% CI for GMC ratio (DAPTACEL/Pentacel) <1.5 and upper limit of 95% CI for differences in rates (DAPTACEL minus Pentacel) <10%].
- ‡ Percent achieving 4-fold rise or GMC Post-Dose 4 Pentacel vaccine not inferior to Post-Dose 4 DAPTACEL vaccine [upper limit of 95% CI for GMC ratio (DAPTACEL/Pentacel) <1.5 and upper limit of 95% CI for differences in rates (DAPTACEL minus Pentacel) <10%].
- § Percent achieving 4-fold rise or GMC Post-Dose 3 Pentacel vaccine not inferior to Post-Dose 3 DAPTACEL vaccine [upper limit of 90% CI for GMC ratio (DAPTACEL/Pentacel) <1.5 and upper limit of 90% CI for differences in rates (DAPTACEL minus Pentacel) <10%].
- ¶ Percent achieving 4-fold rise or GMC Post-Dose 4 Pentacel vaccine not inferior to Post-Dose 4 DAPTACEL vaccine [upper limit of 90% CI for GMC ratio (DAPTACEL/Pentacel) <1.5 and upper limit of 90% CI for differences in rates (DAPTACEL minus Pentacel) <10%].
- # Non-inferiority criterion is not met for GMC Post-Dose 4 Pentacel vaccine relative to Post-Dose 4 DAPTACEL vaccine [upper limit of 90% CI for GMC ratio (DAPTACEL/Pentacel) = 2.25, which exceeds the non-inferiority criterion of <1.5].
N = 143 N = 481-485 N = 113 N = 127-128 Anti-PT
% achieving 4-fold rise*
GMC (EU/mL)
95.8†
102.62†
87.3
61.88
93.8‡
107.89‡
91.3
100.29N = 218-318 N = 714-1,016 N = 230-367 N = 237-347 Anti-FHA
% achieving 4-fold rise*
GMC (EU/mL)
81.9§
73.68§
60.9
29.22
88.4¶
107.94¶
79.3
64.02Anti-PRN
% achieving 4-fold rise*
GMC (EU/mL)
74.2§
36.05§
75.4
43.25
92.7¶
93.59#
98.3
186.07Anti-FIM
% achieving 4-fold rise*
GMC (EU/mL)
91.7§
268.15§
86.3
267.18
93.5¶
553.39¶
91.6
513.5414.4 Poliomyelitis
In Study P3T06 (Table 1), in which infants were randomized to receive the first three doses of Pentacel or DAPTACEL + IPOL + ActHIB at 2, 4, and 6 months of age, one month following the third dose of study vaccines, ≥99.4% of participants in both groups (Pentacel: N = 338-350), (DAPTACEL + IPOL + ActHIB: N = 1,050-1,097) achieved neutralizing antibody levels of ≥1:8 for Poliovirus types 1, 2, and 3.
In Study 494-01 (Table 1), in which infants were randomized to receive Pentacel or HCPDT + POLIOVAX + ActHIB, GMTs (1/dil) of antibodies to Poliovirus types 1, 2, and 3 one month following Dose 4 of Pentacel (N = 851-857) were 2,304, 4,178, and 4,415, respectively, and one month following Dose 4 of POLIOVAX (N = 284-287) were 2,330, 2,840, and 3,300, respectively.
14.5 Invasive Disease due to H. Influenzae Type b
Anti-PRP seroprotection rates and GMCs one month following Dose 3 of Pentacel or separately administered ActHIB in studies 494-01, P3T06, and M5A10 are presented in Table 6. In Study 494-01, non-inferiority criteria were not met for the proportion of participants who achieved an anti-PRP level ≥1.0 mcg/mL and for anti-PRP GMCs following Pentacel compared with separately administered ActHIB. In each of Studies P3T06 and M5A10, the non-inferiority criterion was met for the proportion of participants who achieved an anti-PRP level ≥1.0 mcg/mL following Pentacel compared with separately administered ActHIB. In Study M5A10, the non-inferiority criterion was met for anti-PRP GMCs following Pentacel compared with separately administered ActHIB.
Table 6: Anti-PRP Seroprotection Rates and GMCs One Month Following Three Doses of Pentacel or Separate DTaP + IPV + ActHIB Administered at 2, 4, and 6 Months of Age in Studies 494-01, P3T06, and M5A10 Per Protocol Immunogenicity population for all studies.
IPV indicates Poliovirus Vaccine Inactivated.- * Percent achieving specified level following Pentacel vaccine not inferior to ActHIB vaccine [upper limit of 90% CI for difference in rates (ActHIB minus Pentacel) <10%].
- † Non-inferiority criterion not met for percent achieving anti-PRP ≥1.0 mcg/mL following Pentacel vaccine relative to ActHIB vaccine [upper limit of 90% CI for difference in rates (ActHIB minus Pentacel), 12.9%, exceeds the non-inferiority criterion <10%].
- ‡ Non-inferiority criterion not met for GMC following Pentacel vaccine relative to ActHIB vaccine [upper limit of 90% CI of GMC ratio (ActHIB/Pentacel), 2.26, exceeds the non-inferiority criterion <1.5].
- § Non-inferiority criterion not pre-specified.
- ¶ Percent achieving specified level following Pentacel vaccine not inferior to ActHIB vaccine [upper limit of 95% CI for difference in rates (ActHIB minus Pentacel) <10%].
- # GMC following Pentacel vaccine not inferior to ActHIB vaccine [upper limit of 90% CI of GMC ratio (ActHIB/Pentacel) <1.5].
Study 494-01 Pentacel
N = 1,127HCPDT + POLIOVAX + ActHIB
N = 401% achieving anti-PRP ≥0.15 mcg/mL 95.4* 98.3 % achieving anti-PRP ≥1.0 mcg/mL 79.1† 88.8 Anti-PRP GMC (mcg/mL) 3.19‡ 6.23 Study P3T06 Pentacel
N = 365DAPTACEL + IPOL + ActHIB
N = 1,128% achieving anti-PRP ≥0.15 mcg/mL 92.3* 93.3 % achieving anti-PRP ≥1.0 mcg/mL 72.1* 70.8 Anti-PRP GMC (mcg/mL) 2.31§ 2.29 Study M5A10 Pentacel
N = 826DAPTACEL + IPOL + ActHIB
N = 421% achieving anti-PRP ≥0.15 mcg/mL 93.8¶ 90.3 % achieving anti-PRP ≥1.0 mcg/mL 75.1¶ 74.8 Anti-PRP GMC (mcg/mL) 2.52# 2.38 In Study 494-01, at 15 months of age prior to receipt of Dose 4 of study vaccines, 68.6% of Pentacel recipients (N = 829) and 80.8% of separately administered ActHIB recipients (N = 276) had an anti-PRP level ≥0.15 mcg/mL. Following Dose 4 of study vaccines, 98.2% of Pentacel recipients (N = 874) and 99.0% of separately administered ActHIB recipients (N = 291) had an anti-PRP level ≥1.0 mcg/mL.
In Study P3T06, at 15 months of age prior to receipt of Dose 4 of study vaccines, 65.4% of Pentacel recipients (N = 335) and 60.7% of separately administered ActHIB recipients (N = 323) had an anti-PRP level ≥0.15 mcg/mL. Following Dose 4 of study vaccines, 97.8% of Pentacel recipients (N = 361) and 95.9% of separately administered ActHIB recipients (N = 340) had an anti-PRP level ≥1.0 mcg/mL.
14.6 Concomitantly Administered Vaccines
In Study P3T06, (Table 1) there was no evidence for reduced antibody responses to hepatitis B vaccine (percent of participants with anti-HBsAg ≥10 mIU/mL and GMCs) or PCV7 (percent of participants with antibody levels ≥0.15 mcg/mL and ≥0.5 mcg/mL and GMCs to each serotype) administered concomitantly with Pentacel (N = 321-325) relative to these vaccines administered concomitantly with DAPTACEL + IPOL + ActHIB (N = 998-1,029). The immune responses to hepatitis B vaccine and PCV7 were evaluated one month following the third dose.
In Study 494-03, (Table 1) there was no evidence for interference in the immune response to the fourth dose of PCV7 (percent of participants with antibody levels ≥0.15 mcg/mL and ≥0.5 mcg/mL and GMCs to each serotype) administered at 15 months of age concomitantly with Pentacel (N = 155) relative to this vaccine administered concomitantly with MMR and varicella vaccines (N = 158). There was no evidence for interference in the immune response to MMR and varicella vaccines (percent of participants with pre-specified seroresponse level) administered at 15 months of age concomitantly with Pentacel (N = 154) relative to these vaccines administered concomitantly with PCV7 (N = 144). The immune responses to MMR, varicella vaccine and the fourth dose of PCV7 were evaluated one month post-vaccination.
-
15 REFERENCES
- 1 DAPTACEL® [full prescribing information]. Toronto, ON: Sanofi Pasteur; 2016.
- 2 CDC. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP) regarding routine poliovirus vaccination. MMWR 2009;58:829-30.
- 3 Stratton KR, et al. editors. Adverse events associated with childhood vaccines; evidence bearing on causality. Washington D.C.: National Academy Press. 1994. p. 67-117.
- 4 Braun MM. Report of a US Public Health Service workshop on hypotonic-hyporesponsive episode (HHE) after pertussis immunization. Pediatrics 1998;102(5)1-5.
- 5 Rothstein EP, et al. Comparison of antigenuria after immunization with three Haemophilus influenzae type b conjugate vaccines. Pediatr Infect Dis J 1991;10:311-4.
- 6 Stainer DW. Production of diphtheria toxin. In: Manclark CR, editor. Proceedings of an informal consultation on the World Health Organization requirements for diphtheria, tetanus, pertussis and combined vaccines. United States Public Health Service, Bethesda, MD. DHHS 91-1174. 1991. p. 7-11.
- 7 Mueller JH, Miller PA. Variable factors influencing the production of tetanus toxin. J Bacteriol 1954;67(3):271-7.
- 8 Stainer DW, et al. A simple chemically defined medium for the production of phase 1 Bordetella pertussis. J Gen Microbiol 1971;63:211-20.
- 9 Chu CY, et al. Further studies on the immunogenicity of Haemophilus influenzae type b and pneumococcal type 6A polysaccharide-protein conjugates. Infect Immun 1983;40:245-56.
- 10 Mueller JH, et al. Production of diphtheria toxin of high potency (100 Lf) on a reproducible medium. J Immunol 1941;40:21-32.
- 11 Department of Health and Human Services, Food and Drug Administration. Biological products; bacterial vaccines and toxoids; implementation of efficacy review; proposed rule. Federal Register 1985;50(240):51002-117.
- 12 Vitek CR, Tiwari TS, Wharton M. Diphtheria toxoid. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 7th ed. Philadelphia, PA: W. B. Saunders; 2018:7:261-75.
- 13 Roper M, Wassilak SGF, et al. Tetanus toxoid. In: Plotkin SA, Orenstein WA, Offit PA, editors. Vaccines. 7th ed. Philadelphia, PA: W.B. Saunders; 2018:18:1052-79.
- 14 Sutter RW, et al. Defining surrogate serologic tests with respect to predicting protective vaccine efficacy: Poliovirus vaccination. In: Williams JC, et al. eds. Combined vaccines and simultaneous administration. Current issues and perspectives. New York, NY: The New York Academy of Sciences. 1995:289-99.
- 15 Robbins JB, et al. Quantitative measurement of "natural" and immunization-induced Haemophilus influenzae type b capsular polysaccharide antibodies. Pediatr Res 1973;7:103-10.
- 16 Peltola H, et al. Haemophilus influenzae type b capsular polysaccharide vaccine in children: a double-blind field study of 100,000 vaccinees 3 months to 5 years of age in Finland. Pediatrics 1977;60:730-7.
- 17 Kayhty H, et al. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis 1983;147:1100.
- 18 Anderson P. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J Infect Dis 1984;149:1034.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
The vial stoppers for the DTaP-IPV and ActHIB vaccine components of Pentacel are not made with natural rubber latex.
5 Dose Package (NDC No. 49281-511-05) containing 5 vials of DTaP-IPV component (NDC No. 49281-561-01) to be used to reconstitute 5 single-dose vials of lyophilized ActHIB vaccine component (NDC No. 49281-544-58).
-
17 PATIENT COUNSELING INFORMATION
Before administration of Pentacel, health-care personnel should inform the parent or guardian of the benefits and risks of the vaccine and the importance of completing the immunization series unless a contraindication to further immunization exists.
The health-care provider should inform the parent or guardian about the potential for adverse reactions that have been temporally associated with Pentacel or other vaccines containing similar ingredients. The health-care provider should provide the Vaccine Information Statements (VIS) which are required by the National Childhood Vaccine Injury Act of 1986 to be given with each immunization. The parent or guardian should be instructed to report adverse reactions to their health-care provider.
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - Kit Package
DTaP-IPV/Hib
NDC: 49281-511-05
Diphtheria and Tetanus Toxoids
and Acellular Pertussis Adsorbed,
Inactivated Poliovirus and
Haemophilus b Conjugate
(Tetanus Toxoid Conjugate) Vaccine5 single-dose vials
0.5 mL5 single-dose vials
Rx only
Pentacel®
For children 6 weeks through
4 years of age (prior to 5th birthday)SANOFI PASTEUR

-
PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Label - 561
NDC: 49281-561-01
0.5 mLDTaP-IPV Component
of Pentacel®Not to be used alone.
For use only to reconstitute
ActHIB®.
Rx onlySanofi Pasteur Limited
-
PRINCIPAL DISPLAY PANEL - 0.5 mL Vial Label - 544
NDC: 49281-544-58
Hib
Single dose
6 wks-4 yrs
Haemophilus b Conjugate Vaccine
(Tetanus Toxoid Conjugate)
ActHIB® Component of Pentacel®
Sanofi Pasteur SARx only
-
INGREDIENTS AND APPEARANCE
PENTACEL
diphtheria and tetanus toxoids and acellular pertussis adsorbed, inactivated poliovirus and haemophilus b conjugate (tetanus toxoid conjugate) vaccine kitProduct Information Product Type VACCINE Item Code (Source) NDC: 49281-511 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49281-511-05 1 in 1 PACKAGE Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 5 VIAL, SINGLE-DOSE 2.5 mL Part 2 5 VIAL, SINGLE-DOSE 2.5 mL Part 1 of 2 DTAP-IPV
diphtheria and tetanus toxoids and acellular pertussis adsorbed, inactivated poliovirus injection, suspensionProduct Information Item Code (Source) NDC: 49281-561 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CORYNEBACTERIUM DIPHTHERIAE TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: IRH51QN26H) (CORYNEBACTERIUM DIPHTHERIAE TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:IRH51QN26H) CORYNEBACTERIUM DIPHTHERIAE TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) 15 [Lf] in 0.5 mL CLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: K3W1N8YP13) (CLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:K3W1N8YP13) CLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) 5 [Lf] in 0.5 mL BORDETELLA PERTUSSIS TOXOID ANTIGEN (GLUTARALDEHYDE INACTIVATED) (UNII: F4TN0IPY37) (BORDETELLA PERTUSSIS TOXOID ANTIGEN (GLUTARALDEHYDE INACTIVATED) - UNII:F4TN0IPY37) BORDETELLA PERTUSSIS TOXOID ANTIGEN (GLUTARALDEHYDE INACTIVATED) 20 ug in 0.5 mL BORDETELLA PERTUSSIS FILAMENTOUS HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: 8C367IY4EY) (BORDETELLA PERTUSSIS FILAMENTOUS HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:8C367IY4EY) BORDETELLA PERTUSSIS FILAMENTOUS HEMAGGLUTININ ANTIGEN (FORMALDEHYDE INACTIVATED) 20 ug in 0.5 mL BORDETELLA PERTUSSIS PERTACTIN ANTIGEN (UNII: 63GD90PP8X) (BORDETELLA PERTUSSIS PERTACTIN ANTIGEN - UNII:63GD90PP8X) BORDETELLA PERTUSSIS PERTACTIN ANTIGEN 3 ug in 0.5 mL BORDETELLA PERTUSSIS FIMBRIAE 2/3 ANTIGEN (UNII: 1O0600285A) (BORDETELLA PERTUSSIS FIMBRIAE 2/3 ANTIGEN - UNII:1O0600285A) BORDETELLA PERTUSSIS FIMBRIAE 2/3 ANTIGEN 5 ug in 0.5 mL POLIOVIRUS TYPE 1 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: 0LVY784C09) (POLIOVIRUS TYPE 1 ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:0LVY784C09) POLIOVIRUS TYPE 1 ANTIGEN (FORMALDEHYDE INACTIVATED) 29 [D'ag'U] in 0.5 mL POLIOVIRUS TYPE 2 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: 23JE9KDF4R) (POLIOVIRUS TYPE 2 ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:23JE9KDF4R) POLIOVIRUS TYPE 2 ANTIGEN (FORMALDEHYDE INACTIVATED) 7 [D'ag'U] in 0.5 mL POLIOVIRUS TYPE 3 ANTIGEN (FORMALDEHYDE INACTIVATED) (UNII: 459ROM8M9M) (POLIOVIRUS TYPE 3 ANTIGEN (FORMALDEHYDE INACTIVATED) - UNII:459ROM8M9M) POLIOVIRUS TYPE 3 ANTIGEN (FORMALDEHYDE INACTIVATED) 26 [D'ag'U] in 0.5 mL Inactive Ingredients Ingredient Name Strength ALUMINUM PHOSPHATE (UNII: F92V3S521O) 1.5 mg in 0.5 mL PHENOXYETHANOL (UNII: HIE492ZZ3T) 3.3 mg in 0.5 mL POLYSORBATE 80 (UNII: 6OZP39ZG8H) GLUTARAL (UNII: T3C89M417N) FORMALDEHYDE (UNII: 1HG84L3525) ALBUMIN BOVINE (UNII: 27432CM55Q) STREPTOMYCIN SULFATE (UNII: CW25IKJ202) NEOMYCIN (UNII: I16QD7X297) POLYMYXIN B SULFATE (UNII: 19371312D4) WATER (UNII: 059QF0KO0R) Product Characteristics Color WHITE (WHITE TO OFF-WHITE (YELLOW TINGE)) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49281-561-01 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125145 06/20/2008 Part 2 of 2 ACTHIB
haemophilus b conjugate vaccine (tetanus toxoid conjugate) injectionProduct Information Item Code (Source) NDC: 49281-544 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HAEMOPHILUS INFLUENZAE TYPE B STRAIN 1482 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN (UNII: FLV5I5W26R) (HAEMOPHILUS INFLUENZAE TYPE B STRAIN 1482 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN - UNII:FLV5I5W26R) HAEMOPHILUS INFLUENZAE TYPE B STRAIN 1482 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN 10 ug in 0.5 mL Inactive Ingredients Ingredient Name Strength Sucrose (UNII: C151H8M554) 42.5 mg in 0.5 mL Product Characteristics Color WHITE (WHITE TO OFF-WHITE (YELLOW TINGE)) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49281-544-58 0.5 mL in 1 VIAL, SINGLE-DOSE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125145 06/20/2008 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date BLA BLA125145 06/20/2008 Labeler - Sanofi Pasteur Inc. (086723285) Establishment Name Address ID/FEI Business Operations Sanofi Pasteur Limited 208206623 MANUFACTURE
Trademark Results [PENTACEL]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PENTACEL 78857520 3545276 Live/Registered |
SANOFI PASTEUR INC. 2006-04-10 |
 PENTACEL 78469500 3065270 Dead/Cancelled |
SANOFI PASTEUR LIMITED/SANOFI PASTEUR LIMITEE 2004-08-18 |
 PENTACEL 77103899 3568122 Live/Registered |
SANOFI PASTEUR INC. 2007-02-09 |
 PENTACEL 77103869 3568121 Live/Registered |
SANOFI PASTEUR INC. 2007-02-09 |
 PENTACEL 76416272 not registered Dead/Abandoned |
SANOFI PASTEUR LIMITED/SANOFI PASTEUR LIMITEE 2002-06-03 |
 PENTACEL 75538591 not registered Dead/Abandoned |
Connaught Technology Corporation 1998-08-18 |
 PENTACEL 74584136 not registered Dead/Abandoned |
Connaught Technology Corporation 1994-10-11 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.