SOHONOS- palovarotene capsule
SOHONOS by
Drug Labeling and Warnings
SOHONOS by is a Prescription medication manufactured, distributed, or labeled by Ipsen Biopharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use SOHONOS® safely and effectively. See full prescribing information for SOHONOS.
SOHONOS (palovarotene) capsules, for oral use
Initial U.S. Approval: 2023WARNING: EMBRYO-FETAL TOXICITY and PREMATURE EPIPHYSEAL CLOSURE IN GROWING PEDIATRIC PATIENTS
See full prescribing information for complete boxed warning.- SOHONOS is contraindicated in pregnancy (5.1, 8.1) Because of the risk of teratogenicity and to minimize fetal exposure, SOHONOS is to be administered only if conditions for pregnancy prevention are met (5.1, 8.1)
- SOHONOS causes premature epiphyseal closure in growing pediatric patients with FOP, close monitoring is recommended (5.2, 8.4)
INDICATIONS AND USAGE
SOHONOS is a retinoid indicated for reduction in the volume of new heterotopic ossification in adults and children aged 8 years and older for females and 10 years and older for males with fibrodysplasia ossificans progressiva (FOP) (1).
DOSAGE AND ADMINISTRATION
- Obtain a negative pregnancy test in females of reproductive potential before initiation of SOHONOS (2.1)
- Recommended dosage includes a chronic daily dose, which can be increased for flare-up symptoms (2.2)
- For adults and pediatric patients 14 years and older: Recommended dosage is 5 mg once daily, with an increase in dose at the time of a flare-up to 20 mg once daily for 4 weeks, followed by 10 mg once daily for 8 weeks for a total of 12 weeks (20/10 mg flare-up treatment) (2.2)
- For pediatric patients under 14 years: Weight-adjusted for daily and flare-up dosing. Recommended daily dosage range from 2.5 to 5 mg. Refer to Table 1 in Full Prescribing Information for complete pediatric dosing (2.2)
- Take SOHONOS with food preferably at same time each day (2.3).
- Reduce the dose in the event of adverse reactions as appropriate (2.4)
- See Full Prescribing Information for complete dosing instructions (2)
DOSAGE FORMS AND STRENGTHS
Capsules: 1, 1.5, 2.5, 5, 10 mg (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Premature Epiphyseal Closure: Premature epiphyseal closure occurred with SOHONOS. Assess baseline skeletal maturity before SOHONOS therapy and monitor linear growth in growing pediatric patients (5.2)
- Mucocutaneous Adverse Reactions: Dry skin, lip dry, pruritus, rash, alopecia, erythema, skin exfoliation, and dry eye occurred with SOHONOS. Prevent or treat with skin emollients, sunscreen, artificial tears. Dosage reduction may be required in some patients (2.4, 5.3)
- Metabolic Bone Disorders: Decreased vertebral bone mineral content and bone density may occur. Assess for spinal fracture periodically using radiologic method (5.4)
- Psychiatric Disorders: Depression, anxiety, mood alterations and suicidal thoughts and behaviors occurred with SOHONOS. Contact healthcare provider if new or worsening symptoms develop in patients treated with SOHONOS (5.5)
- Night Blindness: May occur and make driving at night hazardous (5.6)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥10%) are dry skin, lip dry, arthralgia, pruritus, pain in extremity, rash, alopecia, erythema, headache, back pain, skin exfoliation, nausea, musculoskeletal pain, myalgia, dry eye, hypersensitivity, peripheral edema, and fatigue (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact IPSEN Biopharmaceuticals, Inc at 1-855-463-5127 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- CYP3A4 Inhibitors: May increase SOHONOS exposure. Avoid concomitant use of strong/moderate CYP3A4 inhibitors. If concomitant use of moderate CYP3A4 inhibitors is unavoidable, reduce the dose of SOHONOS by half (2.5, 7.1)
- CYP3A4 Inducers: May decrease SOHONOS exposure. Avoid concomitant use of strong/moderate CYP3A4 inducers (7.1)
- Vitamin A: May cause additive effects (7.2)
- Tetracyclines: Avoid concomitant use with SOHONOS (7.3)
- Systemic Corticosteroids: No clinically significant drug interaction is expected with concomitant use of SOHONOS (7.4)
USE IN SPECIFIC POPULATIONS
- Pregnancy: May cause fetal harm (2.1, 4, 8.1)
- Growing pediatric patients are recommended to undergo baseline assessment of growth and skeletal maturity before starting treatment and continued clinical and radiographic monitoring every 6 to 12 months until patients reach skeletal maturity or final adult height (5.2, 8.4)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: EMBRYO-FETAL TOXICITY and PREMATURE EPIPHYSEAL CLOSURE IN GROWING PEDIATRIC PATIENTS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Pregnancy Testing Prior to Treatment with SOHONOS
2.2 Recommended Dosage and Duration
2.3 Administration Instructions
2.4 Dosage Reduction for Adverse Reactions
2.5 Dosage Modifications for Drug Interactions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
5.2 Premature Epiphyseal Closure in Growing Pediatric Patients
5.3 Mucocutaneous Adverse Reactions
5.4 Metabolic Bone Disorders
5.5 Psychiatric Disorders
5.6 Night Blindness
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on SOHONOS
7.2 Vitamin A
7.3 Tetracyclines
7.4 Systemic Corticosteroids
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Chronic/Flare-up Regimen
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: EMBRYO-FETAL TOXICITY and PREMATURE EPIPHYSEAL CLOSURE IN GROWING PEDIATRIC PATIENTS
Embryo-Fetal Toxicity
SOHONOS is contraindicated in pregnancy. SOHONOS may cause fetal harm. Because of the risk of teratogenicity and to minimize fetal exposure, SOHONOS is to be administered only if conditions for pregnancy prevention are met [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
Premature Epiphyseal Closure
Premature epiphyseal closure occurs in growing pediatric patients treated with SOHONOS, close monitoring is recommended [see Warnings and Precautions (5.2) and Use in Specific Populations (8.4)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Pregnancy Testing Prior to Treatment with SOHONOS
For females of reproductive potential, obtain a negative pregnancy test within one week prior to initiating and periodically during SOHONOS therapy. If pregnancy occurs, stop SOHONOS treatment immediately and refer patient to an obstetrician/gynecologist experienced in reproductive toxicity. [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1, 8.3)].
2.2 Recommended Dosage and Duration
Dosage Overview
Take SOHONOS with food preferably at the same time each day [see Dosage and Administration (2.3)]. The recommended dosing for SOHONOS includes a chronic daily dosage (daily dose) which can then be modified/increased in the event of FOP flare-up symptoms (flare-up dose).
Initiate flare-up treatment at the onset of the first symptom indicative of a FOP flare-up or substantial high-risk traumatic event likely to lead to a flare-up (e.g., surgery, intramuscular immunization, mandibular blocks for dental procedures, muscle fatigue, blunt muscle trauma from bumps, bruises, falls, or influenza-like viral illnesses). Symptoms of a FOP flare-up typically include but are not limited to localized pain, soft tissue swelling/inflammation, redness, warmth, decreased joint range of motion, and stiffness.
Recommended Dosage for Adults and Pediatric Patients 14 Years and Older
- Daily Dose: The recommended SOHONOS daily dosage for adults and pediatric patients 14 years and older is 5 mg daily. Stop daily dosing when flare-up dosing begins.
- Flare-up Dose:
- The recommended SOHONOS flare-up dosage for adults and pediatric patients 14 years and older is 20 mg daily for 4 weeks, followed by 10 mg daily for 8 weeks (for a total of 12 weeks of flare-up treatment), even if symptoms resolve earlier, then return to daily dosing of 5 mg.
- If during the course of flare-up treatment, the patient experiences marked worsening of the original flare-up site or another flare-up at a new location, restart the 12-week flare-up dosing at 20 mg daily.
- For flare-up symptoms that have not resolved at the end of the 12-week period, the 10 mg daily dosage may be extended in 4-week intervals and continued until the flare-up symptoms resolve. If new flare-up symptoms occur after the 5 mg daily dosing is resumed, flare-up dosing may be restarted.
Recommended Dosage for Pediatric Patients Aged 8 to 13 Years for Females and Aged 10 to 13 Years for Males
- Daily Dose: The recommended SOHONOS daily dosage for patients under 14 years of age is weight-based ranging from 2.5 mg to 5 mg daily (see Table 1). Stop daily dosing when flare-up dosing begins.
- Flare-up Dose:
- The recommended flare-up SOHONOS dosage for patients under 14 years of age is weight-based (see Table 1). Administer the initial flare-up dosage once daily for 4 weeks, then administer the lower flare-up dosage once daily for 8 weeks (for a total of 12 weeks of flare-up treatment), even if symptoms resolve earlier, then return to daily dosing (see Table 1).
- If during the course of flare-up treatment, the patient experiences marked worsening of the original flare-up site or another flare-up at a new location, restart the 12-week flare-up dosing with the Week 1 to 4 dose.
- For flare-up symptoms that have not resolved at the end of the 12-week period, the Week 5 to 12 flare-up dose may be extended in 4-week intervals and continued until the flare-up symptoms resolve. If new flare-up symptoms occur after daily dosing is resumed, flare-up dosing may be restarted.
Table 1. Recommended SOHONOS Weight-Based Dosage for Pediatric Patients Aged 8 to 13 Years for Females and 10 to 13 Years for Males * Weight Daily Dosage Week 1 to 4 Flare-up Dosage Week 5 to 12 Flare-up Dosage - * once daily
10 kg to 19.9 kg 2.5 mg 10 mg 5 mg 20 kg to 39.9 kg 3 mg 12.5 mg 6 mg 40 kg to 59.9 kg 4 mg 15 mg 7.5 mg ≥ 60 kg 5 mg 20 mg 10 mg 2.3 Administration Instructions
Take SOHONOS with food preferably at the same time each day. SOHONOS may be swallowed whole, or capsules may be opened and the contents emptied onto one teaspoon (5 mL) of soft food (such as apple sauce, low-fat yogurt, or warm oatmeal) and taken within 1 hour of opening provided it was maintained at room temperature and not exposed to direct sunlight [see Clinical Pharmacology (12.3)]. Do not administer with grapefruit, pomelo, or juices containing these fruits.
2.4 Dosage Reduction for Adverse Reactions
If patients experience adverse reactions that require dosage reduction during either the SOHONOS daily dosing or flare-up dosing, reduce the daily dosage to the next lower dose as shown in Table 2 at the discretion of the healthcare provider; reduce the dosage further if adverse reactions do not improve. If the patient is already receiving the lowest possible tolerated dose, then consider discontinuing SOHONOS temporarily or permanently. Initiate subsequent flare-up dosing at the same reduced dose that was tolerated previously.
Table 2. Dose Reduction of SOHONOS for Flare-Up and Chronic Treatment Dose Prescribed Reduced Dose 20 mg 15 mg 15 mg 12.5 mg 12.5 mg 10 mg 10 mg 7.5 mg 7.5 mg 5 mg 6 mg 4 mg 5 mg 2.5 mg 4 mg 2 mg 3 mg 1.5 mg 2.5 mg 1 mg 2.5 Dosage Modifications for Drug Interactions
Moderate CYP3A Inhibitors:
Avoid concomitant use of a moderate CYP3A inhibitor, if possible. If concomitant use will occur, reduce the dose of SOHONOS by half as shown in Table 3 when co-administered with moderate CYP3A inhibitors [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
Table 3. Dose Reduction of SOHONOS for Use with Moderate CYP3A Inhibitors Weight Daily
DosageWeek 1 to 4
Flare-up DosageWeek 5 to 12
Flare-up Dosage- * All pediatric patients ≥14 years of age and adults should receive the dose in the ≥60 kg weight category.
10 kg to 19.9 kg 1 mg 5 mg 2.5 mg 20 kg to 39.9 kg 1.5 mg 6 mg 3 mg 40 kg to 59.9 kg 2 mg 7.5 mg 4 mg ≥ 60 kg* 2.5 mg 10 mg 5 mg - 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
SOHONOS is contraindicated in the following patients:
- During Pregnancy [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
- A history of allergy or hypersensitivity to retinoids, or to any component of SOHONOS. Anaphylaxis and other allergic reactions have occurred with other retinoids. [see Description (11)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Embryo-Fetal Toxicity
SOHONOS can cause fetal harm and is contraindicated during pregnancy. SOHONOS is a member of the retinoid class of drugs which is associated with birth defects in humans. In animal reproduction studies, palovarotene administered orally to pregnant rats during organogenesis was teratogenic and caused fetal malformations typical of retinoids including cleft palate, misshapen skull bones, and shortening of the long bones at clinically relevant exposures.
For females of reproductive potential, verify that the patient is not pregnant prior to initiating treatment, periodically during the course of therapy and one month after treatment discontinuation. Advise females of reproductive potential to use an effective method of contraception at least one month prior to treatment, during treatment with SOHONOS and for 1 month after the last dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.3)]. If a pregnancy occurs during SOHONOS treatment, discontinue treatment immediately and refer the patient to an obstetrician/gynecologist experienced in reproductive toxicity for further evaluation and counseling.
Patients should be informed not to donate blood during SOHONOS therapy and for 1 week following discontinuation because the blood might be given to a pregnant patient whose fetus must not be exposed to palovarotene.
5.2 Premature Epiphyseal Closure in Growing Pediatric Patients
SOHONOS can cause irreversible premature epiphyseal closure and potential adverse effects on growth. In clinical studies, premature epiphyseal closure occurred with SOHONOS treatment in growing pediatric patients with FOP [see Adverse Reactions (6.1) and Use in Specific Populations (8.4)].
Monitoring of linear growth is recommended in growing pediatric patients [see Use in Specific Populations (8.4)]. Prior to starting treatment with SOHONOS, all growing pediatric patients should undergo baseline assessment of skeletal maturity via hand/wrist and knee x-rays, standard growth curves and pubertal staging. Continued monitoring is recommended every 6 to 12 months until patients reach skeletal maturity or final adult height.
If a patient exhibits signs of premature epiphyseal closure or adverse effects on growth based on clinical or radiologic evaluations, further evaluation may be required, including an assessment of the benefits and risks of continued treatment, or temporary or permanent discontinuation of SOHONOS until the patient achieves epiphyseal closure and skeletal maturity.
5.3 Mucocutaneous Adverse Reactions
Mucocutaneous adverse reactions including dry skin, lip dry, pruritus, rash, alopecia, erythema, skin exfoliation [skin peeling], and dry eye occurred in most (98%) patients treated with SOHONOS. SOHONOS may contribute to an increased risk of skin and soft tissue infections, particularly paronychia and decubitus ulcer, due to a decreased skin barrier from adverse reactions such as dry and peeling skin [see Adverse Reactions (6.1)]. Some of these mucocutaneous adverse reactions led to dose reductions which occurred more frequently during flare-up dosing suggesting a dose response relationship.
Prophylactic measures to minimize risk and/or treat the mucocutaneous adverse reactions are recommended (e.g., skin emollients, sunscreen, lip moisturizers, or artificial tears). Some patients may require dose reduction or drug discontinuation [see Dosage and Administration (2.4)].
Photosensitivity
Photosensitivity reactions, such as exaggerated sunburn reactions (e.g., burning, erythema, blistering) involving areas exposed to the sun have been associated with the use of retinoids and may occur with SOHONOS. Precautionary measures for phototoxicity are recommended. Excessive exposure to sun or artificial ultraviolet light should be avoided, and protection from sunlight should be used when exposure cannot be avoided (use of sunscreens, protective clothing, and use of sunglasses).
5.4 Metabolic Bone Disorders
Bone mineral density and fracture
Retinoids are associated with bone toxicity, including reductions in bone mass and spontaneous reports of osteoporosis and fracture. In FOP clinical trials, SOHONOS resulted in decreased vertebral bone mineral content and bone density, and an increased risk of radiologically observed vertebral (T4 to L4) fractures in treated adult and pediatric patients compared to untreated patients. Periodic radiological assessment of the spine is recommended. [see Adverse Reactions (6.1)].
5.5 Psychiatric Disorders
New or worsening psychiatric events were reported with SOHONOS use. These include depression, anxiety, mood alterations and suicidal thoughts and behaviors. There is a relatively high background prevalence of psychiatric disorders in untreated patients with FOP. Monitor for development of new or worsening psychiatric symptoms during treatment with SOHONOS [see Adverse Reactions (6.1)]. Individuals with a history of psychiatric illness may be more susceptible to these adverse effects. Patients and/or caregivers should contact their healthcare provider if new or worsening psychiatric symptoms develop during treatment with SOHONOS.
5.6 Night Blindness
Night blindness has been associated with systemic retinoids, including SOHONOS. This may be dose-dependent, making driving a vehicle at night potentially hazardous during treatment. Night blindness is generally reversible after cessation of treatment but can also persist in some cases. Advise patients to be cautious when driving or operating any vehicle at night and to seek medical attention in the event of vision impairment.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Premature Epiphyseal Closure in Growing Pediatric Patients [see Warnings and Precautions (5.2)]
- Mucocutaneous Adverse Reactions [see Warnings and Precautions (5.3)]
- Metabolic Bone Disorders [see Warnings and Precautions (5.4)]
- Psychiatric Disorders [see Warnings and Precautions (5.5)]
- Night Blindness [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of SOHONOS was evaluated in clinical studies that enrolled a total of 164 subjects with FOP, including 139 subjects in the indicated population of ages 8 years and above for females and 10 years and above for males (8/10 years and older). Most of these subjects received open label treatment with the chronic daily/flare-up regimen, consisting of 5 mg daily dosage of oral SOHONOS with a 20/10 mg dosage as needed for 12 weeks at the time of flare-up (4 weeks of 20 mg once daily followed by 10 mg once daily for 8 weeks), with all doses reduced by weight in subjects who were less than 90% skeletally mature. The mean duration of exposure was 79 weeks for chronic dosing (N=131 subjects) and 35 weeks for flare-up dosing (N=105 subjects). The mean age of these subjects was 19 years (range 8 to 61 years); 51% were male.
Serious adverse reactions occurred in 21 (15%) SOHONOS treated subjects in the 8/10 years or older population with the most common serious adverse reaction being premature epiphyseal closure. Adverse reactions leading to permanent discontinuation occurred in 11 (8%) SOHONOS treated subjects with dry skin being the most common in 2 (1%) subjects. Mucocutaneous adverse reactions leading to dose reductions were more common during SOHONOS 20/10 mg flare-up treatment (37%) than during chronic treatment (4%).
Table 5 below presents adverse reactions which occurred in at least 10% of FOP subjects 8/10 years and older during treatment with chronic or flare-up dosing.
Table 5. Summary of Adverse Reactions Reported at greater than 10% Frequency in FOP Subjects 8/10 years and older in Clinical Trials Adverse Reaction Chronic 5 mg
N=131
n (%)Flare-up dosing 20/10 mg
N=105
n (%)Doses were reduced according to body weight in subjects who were less than 90% skeletally mature - * includes lip dry, chapped lips, cheilitis
- † includes pruritus, pruritus generalized, and rash pruritic
- ‡ includes rash, rash generalized, rash maculo-papular
- § includes erythema, generalized erythema, flushing, rash erythematous
- ¶ includes headache and migraine
- # includes back pain, flank pain, sciatica
- Þ includes myalgia, musculoskeletal discomfort
- ß includes drug eruption, hypersensitivity, pruritus allergic, drug hypersensitivity
- à includes peripheral swelling, edema peripheral
- è includes fatigue, lethargy, asthenia, malaise
Dry skin 80 (61) 60 (57) Lip dry * 62 (47) 40 (38) Arthralgia 47 (36) 32 (31) Pruritus † 45 (34) 50 (48) Pain in extremity 38 (29) 29 (28) Rash ‡ 36 (28) 31 (30) Alopecia 32 (24) 31 (30) Erythema § 25 (19) 34 (32) Headache ¶ 25 (19) 20 (19) Back pain # 22 (17) 12 (11) Skin exfoliation [skin peeling] 20 (15) 30 (29) Nausea 20 (15) 14 (13) Musculoskeletal pain 18 (14) 14 (13) Myalgia Þ 15 (12) 9 (9) Dry eye 13 (10) 23 (22) Hypersensitivity ß 13 (10) 21 (20) Peripheral edema à 12 (9) 20 (19) Fatigue è 7 (5) 12 (11) Premature epiphyseal closure
Subjects under 18 years with open epiphyses were assessed for growth during the clinical studies. Premature epiphyseal closure was identified by scheduled imaging in 27% of subjects who were less than 18 years of age at enrollment and was more common in younger compared with older subjects (31% in subjects between 8/10 years to 14 years and no subjects 14 years or older). Many of the affected subjects exhibited slowing of growth in height. [see Use in Specific Populations (8.4) and Clinical Studies (14)].
Mucocutaneous Adverse Reactions
Mucocutaneous adverse reactions observed in over 10% of subjects (N=134) were dry skin (78%), lip dry (66%), pruritus (55%), alopecia (44%), rash (42%), erythema (37%), skin exfoliation [skin peeling] (31%), and skin irritation (11%). In addition, dry eye occurred in 25% of subjects.
Bone Mineral Density and Fractures
Loss of bone mineral density and radiological vertebral fractures (PT: Spinal fracture) were identified as a risk associated with SOHONOS based on novel analyses performed on whole body CT data in FOP subjects in the Phase 3 study [see Warnings and Precautions (5.4)].
Hepatotoxicity
Retinoids have been associated with dose dependent elevations of liver enzymes and isolated cases of severe hepatitis. In SOHONOS studies of FOP, elevated ALT was observed in 7.0% of subjects during 20/10 mg flare-up dosing and 1% of subjects during 5 mg chronic dosing. There were no subjects who required dose reduction or treatment discontinuation due to liver enzyme elevations.
Hypertriglyceridemia
Systemic retinoids may cause marked elevations of serum triglycerides. In FOP studies, hypertriglyceridemia was reported in 2 subjects during chronic SOHONOS treatment (2%) and in 4 subjects during flare-up dosing (4%).
Pancreatitis
Pancreatitis has been reported with other systemic retinoids, both with and without elevated triglycerides, including fatal cases. In palovarotene studies, one healthy subject developed acute pancreatitis, possibly related to concomitant use of ketoconazole in a drug-drug interaction study. There were no reports of pancreatitis in the FOP clinical studies.
Intracranial Hypertension (Pseudotumor Cerebri)
Systemic retinoid use has been associated with cases of benign intracranial hypertension (also called pseudotumor cerebri), some of which involved the concomitant use of tetracyclines. There were no reports of benign intracranial hypertension in the FOP clinical studies [see Drug Interactions (7.3)].
-
7 DRUG INTERACTIONS
7.1 Effect of Other Drugs on SOHONOS
Clinically significant drug interactions affecting the exposure of SOHONOS are listed in Table 6.
Table 6. Drugs that affect exposure of SOHONOS. Strong CYP3A Inhibitors Clinical Impact Co-administration of SOHONOS with strong CYP3A4 inhibitors increased the exposures of palovarotene [see Clinical Pharmacology (12.3)], which may increase the risk of SOHONOS adverse reactions. Prevention or Management Avoid concomitant use of a strong CYP3A4 inhibitor during SOHONOS treatment [see Dosage and Administration (2.5)]. Moderate CYP3A Inhibitors Clinical Impact Co-administration of SOHONOS with moderate CYP3A4 inhibitors may increase the exposure of palovarotene [see Clinical Pharmacology (12.3)], which may increase the risk of SOHONOS adverse reactions. Prevention or Management Avoid concomitant use of a moderate CYP3A4 inhibitor with SOHONOS, if possible.
If co-administration will occur, reduce the SOHONOS dose by half when co-administered with moderate CYP3A inhibitors [see Dosage and Administration (2.5)].Strong CYP3A Inducers Clinical Impact Co-administration of SOHONOS with strong CYP3A4 inducers decreased the exposure of palovarotene [see Clinical Pharmacology (12.3)], which may reduce the effectiveness of SOHONOS. Prevention or Management Avoid concomitant use of strong CYP3A4 inducers with SOHONOS. [see Dosage and Administration (2.5)]. Moderate CYP3A Inducers Clinical Impact Co-administration of moderate CYP3A4 inducers with palovarotene may decrease palovarotene exposure [see Clinical Pharmacology (12.3)], which may reduce the effectiveness of SOHONOS. Prevention or Management Avoid concomitant use of moderate CYP3A4 inducers with SOHONOS. 7.2 Vitamin A
Palovarotene belongs to the same pharmacological class as vitamin A. Therefore, the use of both vitamin A and SOHONOS at the same time may lead to additive effects. Concomitant administration of vitamin A in doses higher than the recommended daily allowance (RDA) and/or other oral retinoids with SOHONOS must be avoided because of the risk of hypervitaminosis A.
7.3 Tetracyclines
Systemic retinoid use has been associated with cases of benign intracranial hypertension (also called pseudotumor cerebri), some of which involved the concomitant use of tetracyclines. Avoid coadministration of SOHONOS with tetracycline derivatives [see Adverse Reactions (6.1)].
7.4 Systemic Corticosteroids
No clinically significant drug-drug interaction is expected when SOHONOS is co-administered with prednisone [see Clinical Pharmacology (12.3)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
SOHONOS is contraindicated during pregnancy. Based on the findings in animal studies and class effects of retinoids, SOHONOS can cause fetal harm when administered during pregnancy [see Warnings and Precautions (5.1) and Use in Specific Populations (8.3)]. In animal reproduction studies, oral administration of palovarotene to pregnant rats during the period of organogenesis resulted in multiple fetal malformations typical of retinoids (e.g., cleft palate, malformed skull bone, shortening of the long bones) at doses ≥0.25 mg/kg/day (less than the clinical exposure) (see Data). There are no available human data on SOHONOS use in pregnant women. If pregnancy occurs during treatment with SOHONOS, discontinue treatment immediately and refer the patient to an obstetrician/gynecologist or other specialist experienced in reproductive toxicity for further evaluation and counseling.
Data
Animal Data
Palovarotene oral administration to pregnant rats during the period of organogenesis (gestation day 6 to 17) at doses of 0.01, 0.25 and 1.25 mg/kg/day resulted in fetal malformations consistent with retinoid-mediated embryopathy. Palovarotene exposure resulted in fetal external, visceral and skeletal malformations typical of retinoids, including defects in the mouth (cleft palate, protruding tongue), eye (anophthalmia, microphthalmia), skull (dilated cerebral ventricle, misshapen brain), skeleton (shortening of the long bones), blood vessels, kidney, and ureters at doses ≥ 0.25 mg/kg/day (less than the clinical exposure). The fetal toxicity was observed at maternal rat exposures well below the range of clinically relevant exposures.
8.2 Lactation
Risk Summary
There are no data available on the presence of palovarotene or its main metabolites in either animal or human milk, the effects on the breastfed infant, or on milk production. Because of the potential for serious adverse reactions in breastfed infants exposed to palovarotene through breastmilk, advise females that breastfeeding is not recommended during treatment with SOHONOS, and for at least 1 month after the final dose of SOHONOS.
8.3 Females and Males of Reproductive Potential
SOHONOS can cause fetal harm when administered during pregnancy [see Use in Specific Populations (8.1)]
Pregnancy Testing
Obtain a negative serum pregnancy test within one week prior to SOHONOS therapy. Verify that patient is not pregnant periodically, as needed, over the course of treatment with SOHONOS and one month after treatment discontinuation unless they are not at risk of pregnancy.
Contraception
Females
SOHONOS can cause embryo-fetal harm when administered during pregnancy [see Warnings and Precautions (5.1), Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception at least one month prior to treatment, during treatment with SOHONOS and for 1 month after the last dose, unless continuous abstinence is chosen.
Males
Palovarotene is present in semen (0.7 ng/mL) in amounts 100-fold lower than the maternal plasma exposure at the no observed adverse effect level (NOAEL) for fetal toxicity observed in animal studies. Administration of SOHONOS to a male patient is considered unlikely to affect development of an embryo or fetus carried by a pregnant female sexual partner exposed to SOHONOS via the patient's semen.
8.4 Pediatric Use
The safety and effectiveness of SOHONOS for the treatment of FOP have been established in pediatric patients aged 8 years and older for females and 10 years and older for males. Use of SOHONOS for this indication is supported by evidence from clinical studies in adults and pediatric subjects [see Clinical Studies (14)]. The safety and effectiveness of SOHONOS for the treatment of FOP have not been established in pediatric patients less than 8 years of age in females and less than 10 years of age for males. SOHONOS is not recommended for use in patients younger than 8 years of age for females and 10 years of age for males because of the potential for premature epiphyseal closure. Clinical studies have shown that growing patients with open epiphyses are at risk of developing premature epiphyseal closure when treated with SOHONOS [see Warnings and Precautions (5.2), Adverse Reactions (6.1) and Clinical Studies (14.1)].
Bone Safety
In clinical studies with SOHONOS, assessments of growth and bone safety in growing children included linear and knee height, femur and tibia length measured by Whole-Body Computed Tomography (WBCT), and hand/wrist and knee radiographs. Premature epiphyseal closure has been identified as an irreversible serious risk associated with SOHONOS treatment. Premature epiphyseal closure was observed as early as 6 months after initiating therapy with the majority occurring at or after 12 months. In SOHONOS treated subjects there was a trend of declining height Z-scores in adolescent subjects, potentially due to a loss of linear height and/or increasing spinal deformity. The long-term effects on final height in subjects with FOP treated with SOHONOS have not been established. [see Adverse Reactions (6.1)].
Monitoring Recommendation
Prior to starting treatment with SOHONOS, all growing children should undergo baseline clinical and radiological assessments including but not limited to an assessment of skeletal maturity via hand/wrist and knee x-rays, standard growth curves and pubertal staging. Continued monitoring is recommended every 6-12 months until patients reach skeletal maturity (e.g., epiphyseal closure) or final adult height [see Warnings and Precautions (5.2)].
Juvenile Animal Data
A juvenile animal study was conducted in juvenile rats given daily oral doses of palovarotene at 0.1, 0.5 or 1.2 mg/kg/day from Week 3 to Week 9 of age (prior to epiphyseal fusion). Palovarotene adversely affected skeletal growth and development including, reduction in bone size, abnormal bone shape and/or geometry, diffuse bone loss, and growth in general were affected at ≥ 0.5 mg/kg/day (less than the clinical exposure). As expected, physes were either widened due to an expanded zone of cartilage hypertrophy/maturation (sometimes accompanied by chondrodysplasia), narrowed, or partially/completely closed. In the proximal femur, avascular necrosis of the femoral head was observed accompanied with malformations and microfractures of trabeculae in a few rats at 1.2 mg/kg/day (less than the clinical exposure). In vertebrae, palovarotene completely inhibited the endochondral ossification that normally occurs in the hyaline cartilage at the end of the vertebral body There also were tibial fractures in two high-dose females. The skeletal effects showed evidence of reversing after dosing discontinuation at 0.5 mg/kg/day, but not at highest dose of 1.2 mg/kg/day.
8.5 Geriatric Use
Clinical studies of SOHONOS did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
The effect of renal impairment on the pharmacokinetics of palovarotene has not been evaluated. Given that palovarotene is hepatically eliminated, no dose adjustment of SOHONOS is recommended in patients with mild (CLcr 60 to 89 mL/min) or moderate (CLcr 30 to 59 mL/min) renal impairment. Use of SOHONOS in patients with severe (CLcr 15 to 29 mL/min) renal impairment is not recommended.
8.7 Hepatic Impairment
The effect of moderate or severe hepatic impairment on the pharmacokinetics of palovarotene has not been evaluated. SOHONOS undergoes extensive hepatic metabolism. No dose adjustment is recommended in patients with mild (Child-Pugh A) hepatic impairment. Use of SOHONOS in patients with moderate (Child-Pugh B) or severe (Child-Pugh C) hepatic impairment is not recommended.
-
10 OVERDOSAGE
No clinical experience with an overdose of SOHONOS has been reported. SOHONOS is a derivative of vitamin A. In case of accidental overdose, signs of hypervitaminosis A could appear, including severe headache, nausea or vomiting, drowsiness, irritability and pruritus. Any overdose should be treated with supportive care according to the signs and symptoms exhibited by the patient.
-
11 DESCRIPTION
Palovarotene is an orally bioavailable retinoid that acts as a retinoic acid receptor (RAR) agonist with particular selectivity at the gamma subtype of RAR. Palovarotene is chemically described as 4-[(E)-2-(5,5,8,8-tetramethyl-3-pyrazol-1-ylmethyl-5,6,7,8-tetrahydro-naphthalen-2-yl)-vinyl]-benzoic acid with an empirical formula of C27H30N2O2 and has a molecular weight of 414.54 g/mol.
The structural formula is represented below:
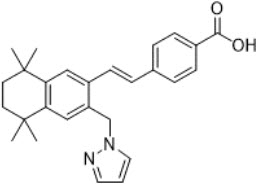
SOHONOS capsules are supplied in 1 mg, 1.5 mg, 2.5 mg, 5 mg and 10 mg strengths. Each capsule contains palovarotene as the active ingredient and the following inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and sodium lauryl sulfate. The capsule consists of gelatin and titanium dioxide. The black printing ink consists of black iron oxide, potassium hydroxide, propylene glycol and Shellac.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
In patients with FOP, abnormal bone formation, including heterotopic ossification (HO), is driven by a gain-of-function mutation in the bone morphogenetic protein (BMP) type I receptor ALK2 (ACVR1). Palovarotene is an orally bioavailable retinoic acid receptor agonist, with particular selectivity at the gamma subtype of RAR. Through binding to RARγ, palovarotene decreases the BMP/ALK2 downstream signaling pathway by inhibiting the phosphorylation of SMAD1/5/8, which reduces ALK2/SMAD-dependent chondrogenesis and osteocyte differentiation resulting in reduced endochondral bone formation.
12.3 Pharmacokinetics
Palovarotene exposure (AUC) increases proportionally from 0.02 to 50 mg (0.001 to 2.5 times the maximum recommended dosage). Steady-state is achieved by Day 3 following once daily dosing. Palovarotene exposure in patients with FOP are listed in Table 7.
Table 7. Palovarotene Exposures in Patients with FOP Palovarotene Dosage Cmax,ss
(ng/mL)AUC0-t
(ng*h/mL)Accumulation Ratio Pharmacokinetics parameters are presented as mean (± SD). Chronic dose 5 mg or weight-based equivalent 40.6 (± 16.2) 264 (± 98.4) 1.16 Flare-up dose 10 mg or weight-based equivalent 78.4 (± 33.3) 540 (± 226) 1.14 Flare-up dose 20 mg or weight-based equivalent 165 (± 72.7) 1060 (± 449) 1.04 Absorption
The median time to achieve peak concentration (Tmax) of palovarotene was 3.0 to 4.0 hours across the chronic dose of 5 mg to flare-up dose of 10 and 20 mg.
Effect of Food
Palovarotene mean AUC and mean Cmax increased by approximately 40% and 16%, respectively; Tmax was delayed by approximately 2 hours with a high-fat, high-calorie meal (800 to 1000 calories, 15% protein, 25% carbohydrate, and 50 to 60% fat).
No clinically significant differences in the AUC and Cmax of palovarotene were observed when palovarotene was administered whole compared to the contents sprinkled onto one teaspoon of applesauce following a high-fat, high-caloric breakfast.
Distribution
The mean (SD) apparent volume of distribution (Vd/F) is 237 (± 90.1) L following administration of a single 20 mg dose with food. Protein binding of palovarotene is 97.9% to 99.6% in vitro.
The mean blood-to-plasma ratio of palovarotene in humans is 0.62.
Elimination
The mean elimination half-life is 8.7 hours following administration of a 20 mg once daily dosage for 14 days with a standard breakfast (800 to 1000 calories, 15% protein, 25% carbohydrate, and 50 to 60% fat). The apparent total body clearance (CL/F) of palovarotene is estimated at 19.9 L/h.
Metabolism
Palovarotene is extensively metabolized by CYP3A4 and to a minor extent by CYP2C8 and CYP2C19.
Following administration of [14-C]-radiolabeled palovarotene, the contribution of palovarotene and its four known major metabolites (M2, M3, M4a, and M4b) represented collectively 40% of the total exposure in plasma. The pharmacological activity of M3 and M4b is approximately 1.7% and 4.2% of the activity of the parent drug.
Specific Populations
There were no clinically significant differences in the pharmacokinetics of palovarotene based on age (2 to 85 years old), sex, race (Asian, black, white and others), smoking status, mild to moderate renal impairment, or mild hepatic impairment. The effect of severe renal impairment, or moderate to severe hepatic impairment on the pharmacokinetics of palovarotene is unknown.
Drug Interaction Studies
Clinical Studies and Model-Informed Approaches
Strong CYP3A Inhibitor: Co-administration of palovarotene with ketoconazole (strong CYP3A4 inhibitor) increased the Cmax and AUC of palovarotene by 2 and 3-fold, respectively.
Moderate CYP3A Inhibitor: Co-administration of palovarotene with erythromycin (moderate CYP3A4 inhibitor) increased the Cmax and AUC of palovarotene by 1.6 and 2.5-fold, respectively.
In Vitro Studies
Cytochrome P450 (CYP) Enzymes: Palovarotene is an inducer of CYP3A4 and CYP2B6, but not CYP1A2, CYP2C8, CYP2C9 and CYP2C19. Palovarotene is not an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 and CYP3A4.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Long term studies to assess the carcinogenic potential of palovarotene have not been conducted.
Palovarotene and its metabolites were negative in the vitro bacterial reverse mutation (Ames) assay and an in vitro micronucleus assay in primary human lymphocyte. Palovarotene did not have any clastogenic effect in the in vivo mouse micronucleus study.
Impairment of Fertility
Palovarotene effects on fertility and reproductive function were assessed in male and female rats. In a female rat fertility study, palovarotene was orally administered to females for 14 days prior to mating with drug naïve males and up to GD 7 at the dose levels of 0.3, 1 and 3 mg/kg/day. Palovarotene caused prolonged periods of diestrous and reduced ovulation rate, resulting in lower numbers of implantation sites and live embryos at 3 mg/kg/day, a dose associated with maternal toxicity.
In a male rat fertility study, palovarotene was orally administered prior to mating, during mating, and up to scheduled euthanasia (approximately 11 weeks in total) at 0.3, 1 and 3 mg/kg/day. Palovarotene did not cause adverse effects on mating, fertility indices, conception rate, reproductive organ weights or sperm parameters up to 1 mg/kg/day (less than the clinical exposure). Males did not tolerate 3 mg/kg/day, as it produced severe systemic toxicity including deaths, adverse skin and hair coat clinical signs, and substantially reduced body weight.
-
14 CLINICAL STUDIES
14.1 Chronic/Flare-up Regimen
Study PVO-1A-301 (NCT03312634, Study 301) was a single arm study in 97 subjects with FOP with R206H mutation aged 4 years and older utilizing the Natural History Study (NHS, PVO-1A-001) as an external control (n=101). The primary efficacy endpoint was annualized volume of new heterotopic ossification (HO) as assessed by low-dose, whole body CT (WBCT) imaging (excluding head). All WBCT images from treated subjects in the 301 study and untreated subjects in the NHS were read in a manner blinded to study origination.
Study 301 subjects received SOHONOS 5 mg daily with increased dosing at the time of a flare-up defined as at least one symptom (e.g. pain, swelling, redness) consistent with a previous flare-up or a substantial high-risk traumatic event likely to lead to a flare-up, to 20 mg once daily for 4 weeks followed by 10 mg once daily for 8 weeks (denoted as the chronic/flare-up regimen), with flare-up treatment extension in 4-week increments for persistent symptoms. Anytime during flare-up treatment, the 12-week treatment restarted if the subject had another flare-up or substantial high-risk traumatic event. The dosing was adjusted according to body weight in skeletally immature children (children who had not reached at least 90% skeletal maturity defined as a bone age of ≥12 years 0 months for girls and ≥14 years 0 months for boys).
The mean age of subjects in the SOHONOS group (N=97) was 15.1 years; and 17.8 years in the untreated group (N=101). There were more male than female subjects in both the SOHONOS (53% and 47%, respectively) and untreated (55% and 45%, respectively) groups.
The mean annualized new HO was 9.4 cm3/year in subjects receiving the chronic/flare-up SOHONOS treatment and 20.3 cm3/year in untreated subjects in the NHS based on a linear mixed effect model. The treatment effect was about 10.9 cm3/year with 95% confidence interval (-21.2 cm3/year, -0.6 cm3/year).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
SOHONOS is an opaque white elongated hard-gelatin capsule. SOHONOS is available in size "0" capsule and supplied as a blister strip containing 14 capsules in a child resistant carton. Capsule contains white to off-white powder. Table 8 shows capsules' strengths, imprints, and NDC numbers.
Table 8. Capsules' Strengths, Imprints, and NDC numbers Strength (mg) Imprint NDC 1 PVO 1 15054-0010-1 1.5 PVO 1.5 15054-0015-1 2.5 PVO 2.5 15054-0025-1 5 PVO 5 15054-0050-1 10 PVO 10 15054-0100-1 Storage and Handling
This package is child-resistant. Keep out of reach of children. Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room temperature]. SOHONOS must be kept in the original carton to protect from light.
SOHONOS capsules may be opened and the contents emptied on a teaspoon of soft food and taken immediately. If not taken immediately, it can be taken after a maximum of one hour after the sprinkling, provided it was maintained at room temperature and not exposed to direct sunlight.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide).
Embryo-Fetal Toxicity:
Advise patients that SOHONOS can cause fetal harm and is contraindicated during pregnancy. Advise patients to verify that they are not pregnant prior to initiating and periodically during SOHONOS treatment as well as one month after treatment discontinuation [see Contraindications (4), Warnings and Precautions (5.1), and Use in Specific Populations (8.3)].
- Advise females of reproductive potential that they must avoid pregnancy while taking SOHONOS and for at least one month following discontinuation of therapy.
- Advise females of childbearing potential to use at least one highly effective method of contraception (i.e. IUD) or two effective methods (i.e. combined hormonal contraception in combination with another method of contraception such as a barrier method) during treatment with SOHONOS [see Use in Specific Populations (8.3)].
- Instruct patients to immediately stop taking SOHONOS if she becomes pregnant while taking SOHONOS and to rapidly consult her healthcare provider if there is a risk of pregnancy or if she might be pregnant.
- Instruct patients to not donate blood during SOHONOS treatment and for 1 week following discontinuation to avoid blood donation to a pregnant patient and fetus.
Lactation
Because of the potential for serious adverse reactions from SOHONOS in a breastfed child, advise females not to breastfeed during treatment with SOHONOS, and for at least 1 month after the last dose of SOHONOS.
Premature Epiphyseal Closure:
Inform patients that SOHONOS has been shown to cause premature epiphyseal closure in growing pediatric patients with FOP and discuss the proposed monitoring plan with the patient and caregiver [see Warnings and Precautions (5.2)].
Mucocutaneous Adverse Reactions:
Advise patients that they may experience dry skin, lip dry, pruritus, rash, alopecia, erythema, skin exfoliation, and dry eye. Discuss the plan to assess their symptoms and adjust the dose if needed [see Dosage and Administration (2.4) and Warning and Precautions (5.3)]. Recommend prophylactic measures to minimize risk and/or treat the mucocutaneous adverse reactions are recommended (e.g. skin emollients, sunscreen, lip moisturizers, artificial tears) [see Warnings and Precautions (5.3)].
Photosensitivity:
Advise patients of potential increased skin sensitivity to sunlight while taking SOHONOS and to minimize exposure to sunlight and artificial ultraviolet light [see Warnings and Precautions (5.3)].
Radiological Vertebral Fractures:
Inform patients that SOHONOS resulted in decreased vertebral bone mineral content, bone density and bone strength as well as an increased risk of radiologically observed vertebral fractures and that periodic radiological assessment of the spine is recommended [see Warnings and Precautions (5.4)].
Psychiatric Disorders:
Advise patients of the possibility of experiencing new or worsening psychiatric effects, including suicidal thoughts and behaviors, depression, depression aggravated, anxiety, and mood alterations. Particular care should be taken in patients with a history of psychiatric illness. Patients should be monitored for signs of depression and referred for appropriate treatment if necessary [see Warnings and Precautions (5.5)].
Night Blindness:
Advise patients of the risk of experiencing night blindness [see Warnings and Precautions (5.6)].
Drug Interactions:
Advise patients to inform their healthcare providers of all concomitant medications, including prescription medicines, over-the-counter drugs, and herbal products [see Dosage and Administration (2.5) and Drug Interactions (7)].
Dosing Instructions:
Advise the patient to take SOHONOS capsules with food. If the patient is unable to swallow the capsule, the capsule contents may be emptied onto soft food [see Dosage and Administration (2.3)].
Missed dose:
If a dose is missed, it should be taken as soon as the patient remembers. If the dose has been missed by more than 6 hours, advise the patient to skip the missed dose and continue with the next scheduled dose. Advise the patient to not take two doses at the same time or in the same day.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
This Medication Guide has been approved by the U.S. Food and Drug Administration Revised: 3/2025 MEDICATION GUIDE
SOHONOS [so-ho-nos]
(palovarotene)
capsules, for oral useWhat is the most important information I should know about SOHONOS? -
SOHONOS can cause birth defects (deformed babies) if taken during pregnancy. Females who are pregnant or who plan to become pregnant must not take SOHONOS.
Females who can become pregnant:- - Your healthcare provider will ask you to take a pregnancy test 1 week before starting treatment with SOHONOS, periodically during treatment, and 1 month after you stop treatment with SOHONOS.
- - You must use effective birth control (contraception) starting at least 1 month before starting treatment with SOHONOS, during treatment and for 1 month after the last dose. Talk to your healthcare provider about birth control methods that may be right for you.
- - If you become pregnant or think you may be pregnant during treatment with SOHONOS, stop taking SOHONOS and call your healthcare provider right away.
- SOHONOS can cause bone growth changes. Children may stop growing while taking SOHONOS. Bone growth changes such as permanent early closure of the growth plate in growing children have happened with SOHONOS. Your healthcare provider will closely monitor your child's bone growth and height during treatment with SOHONOS.
What is SOHONOS? - SOHONOS is a prescription medicine used to reduce the amount of new heterotopic ossification in adults and children 8 years of age and older for females and 10 years of age and older for males with fibrodysplasia ossificans progressiva (FOP).
- SOHONOS is not recommended for females younger than 8 years of age or males younger than 10 years of age.
Do not take SOHONOS if you: - are pregnant. (See "What is the most important information I should know about SOHONOS?")
- are allergic to medicines known as retinoids or any of the ingredients in SOHONOS. See the end of this Medication Guide for a complete list of ingredients in SOHONOS.
Before taking SOHONOS, tell your healthcare provider about all your medical conditions, including if you: - have bone loss (osteoporosis), weak bones or any other bone problems.
- have or had mental health problems.
- have or have had kidney problems.
- have or have had liver problems.
- are breastfeeding or plan to breastfeed. It is not known if SOHONOS passes into your breastmilk. Breastfeeding is not recommended during treatment with SOHONOS and for at least 1 month after the last dose of SOHONOS. Talk to your healthcare provider about the best way to feed your baby if you take SOHONOS.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. SOHONOS and certain other medicines can interact with each other, sometimes causing serious side effects. Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine. How should I take SOHONOS? - Take SOHONOS exactly as your healthcare provider tells you.
- Take SOHONOS capsules whole with food. If you are unable to swallow the capsule, the capsule contents may be emptied onto a teaspoon (5 mL) of soft food (such as apple sauce, low-fat yogurt, or warm oatmeal) and taken within 1 hour of opening.
- Take SOHONOS at about the same time each day.
- Do not eat grapefruit or drink grapefruit juice during treatment with SOHONOS. Grapefruit may increase the levels of SOHONOS in your blood.
- If you miss a dose of SOHONOS, take it as soon as you remember. If the dose has been missed by more than 6 hours, the missed dose should be skipped, and next dose should be taken at the regular time. Do not take two doses at the same time or in the same day.
- Your healthcare provider may change your dose of SOHONOS as needed if you get certain side effects.
- If you take more SOHONOS than prescribed you may have symptoms such as severe headache, nausea or vomiting, drowsiness, irritability, and itching.
What should I avoid while taking SOHONOS? - Do not get pregnant while taking SOHONOS. See "What is the most important Information I should know about SOHONOS?"
- Avoid excessive exposure to sunlight and ultraviolet lights (e.g., tanning machines). SOHONOS may make your skin more sensitive to sunlight and ultraviolet light and you may burn more easily. You should use sunscreen and wear sunglasses and protective clothing that covers your skin to help protect against sunburn if you must be in the sunlight during treatment with SOHONOS.
- Avoid driving at night until you know if SOHONOS has affected your vision. SOHONOS may decrease your ability to see in the dark.
- Do not donate blood while taking SOHONOS and for 1 week after stopping SOHONOS.
What are possible side effects of SOHONOS? SOHONOS can cause serious side effects, including: - See "What is the most important Information I should know about SOHONOS?"
- skin-related problems. SOHONOS may cause skin-related problems including dry skin, lips and eyes, hair loss, itching, redness, rash, and skin peeling. You may be at increased risk of developing skin and soft tissue infections while taking SOHONOS. If you develop these symptoms, your healthcare provider may tell you to use a moisturizer, sunscreen, or artificial tears.
- bone mineral density problems. SOHONOS can cause a reduction in bone mineral density (bone thinning) which can increase the risk of fractures in adults and children. Your healthcare provider should check you for this during treatment with SOHONOS.
- new or worsening mental health problems. SOHONOS may cause new or worsening mental health problems that include depression, anxiety, mood changes, and suicidal thoughts and behaviors. If you have a history of mental health problems, you may be at a higher risk of developing these side effects. Call your healthcare provider if you develop new or worsening mental health symptoms during treatment with SOHONOS. Your healthcare provider should monitor you for signs of depression and refer you for appropriate treatment, if necessary.
- vision problems. Decreased vision in the dark (night blindness). You may have difficulty seeing at night or in low lit areas. Your healthcare provider should send you to see an eye specialist if you experience vision problems.
The most common side effects of SOHONOS include: - dry skin
- dry lips
- hair loss
- itching
- redness
- rash
- skin peeling
- drug eruption
- skin irritation
- swelling and small cracks in corner of the mouth
- nausea
- muscle and joint pain
- dry eyes
- headache
- fatigue
These are not all the possible side effects of SOHONOS. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store SOHONOS? - Store SOHONOS at room temperature between 68° to 77°F (20° to 25°C) in the original carton to protect from light.
- Keep SOHONOS and all medicines out of the reach of children.
General information about the safe and effective use of SOHONOS Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use SOHONOS for a condition for which it was not prescribed. Do not give SOHONOS to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about SOHONOS that is written for healthcare professionals. What are the ingredients in SOHONOS? Active ingredient: palovarotene Inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, microcrystalline cellulose, povidone, and sodium lauryl sulfate. The capsule consists of gelatin and titanium dioxide. The black printing ink consists of black iron oxide, potassium hydroxide, propylene glycol, and Shellac. Distributed by: Ipsen Biopharmaceuticals, Inc. Cambridge, MA; 02142. For more information about SOHONOS, call 855-463-5127 or go to www.SOHONOS.com or drugsafety.USA@ipsen.com SOHONOS is a Registered trademark of Clementia Pharmaceuticals Inc. © 2025 Ipsen Biopharmaceuticals, Inc. All rights reserved. -
SOHONOS can cause birth defects (deformed babies) if taken during pregnancy. Females who are pregnant or who plan to become pregnant must not take SOHONOS.
-
PRINCIPAL DISPLAY PANEL - 1 mg Capsule Blister Pack Carton
NDC: 15054-0010-1
Rx Onlysohonos™
(palovarotene) capsules1 mg
per capsuleFor Oral Use
14 capsulesCAUSES BIRTH DEFECTS
DO NOT GET PREGNANTLIFT OPEN HERE
PHARMACIST:
Dispense in the original
carton with the enclosed
Medication Guide.IPSEN
Innovation for patient care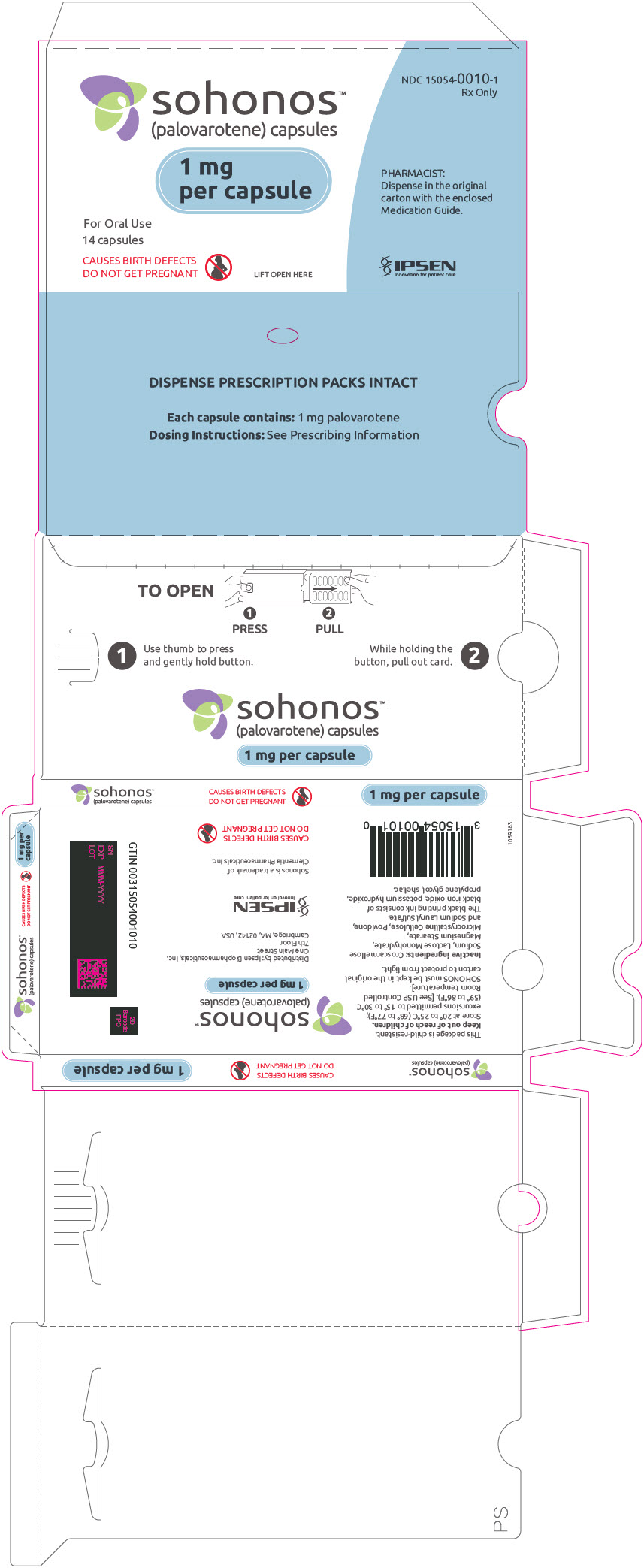
-
PRINCIPAL DISPLAY PANEL - 1.5 mg Capsule Blister Pack Carton
NDC: 15054-0015-1
Rx Onlysohonos™
(palovarotene) capsules1.5 mg
per capsuleFor Oral Use
14 capsulesCAUSES BIRTH DEFECTS
DO NOT GET PREGNANTLIFT OPEN HERE
PHARMACIST:
Dispense in the original
carton with the enclosed
Medication Guide.IPSEN
Innovation for patient care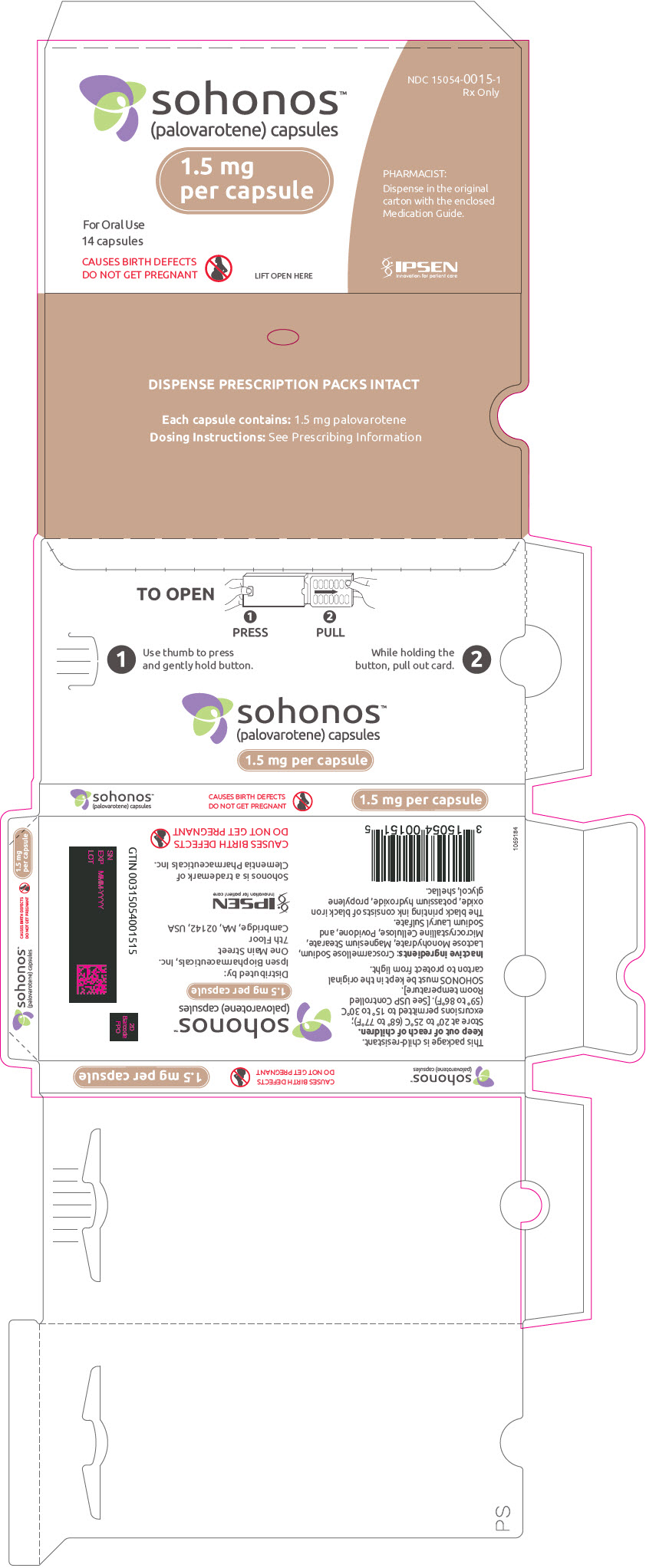
-
PRINCIPAL DISPLAY PANEL - 2.5 mg Capsule Blister Pack Carton
NDC: 15054-0025-1
Rx Onlysohonos™
(palovarotene) capsules2.5 mg
per capsuleFor Oral Use
14 capsulesCAUSES BIRTH DEFECTS
DO NOT GET PREGNANTLIFT OPEN HERE
PHARMACIST:
Dispense in the original
carton with the enclosed
Medication Guide.IPSEN
Innovation for patient care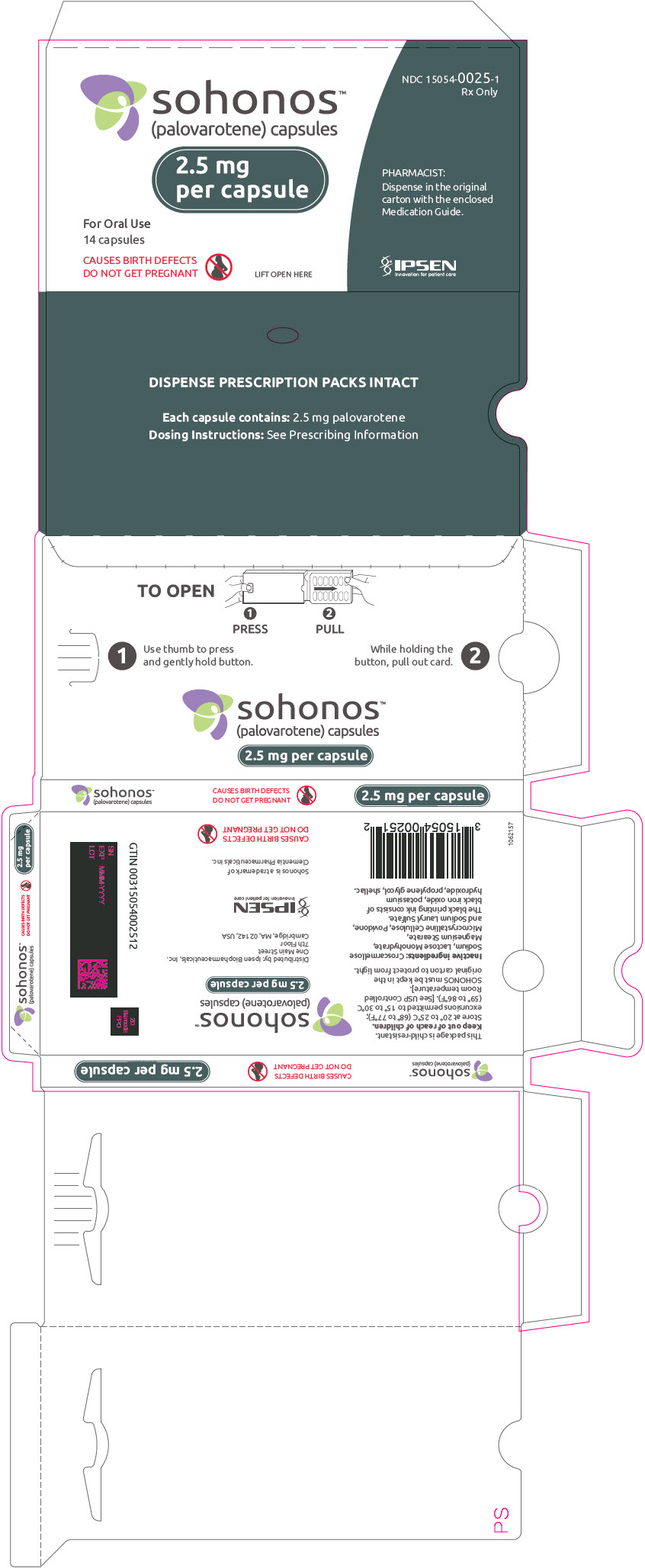
-
PRINCIPAL DISPLAY PANEL - 5 mg Capsule Blister Pack Carton
NDC: 15054-0050-1
Rx Onlysohonos™
(palovarotene) capsules5 mg
per capsuleFor Oral Use
14 capsulesCAUSES BIRTH DEFECTS
DO NOT GET PREGNANTLIFT OPEN HERE
PHARMACIST:
Dispense in the original
carton with the enclosed
Medication Guide.IPSEN
Innovation for patient care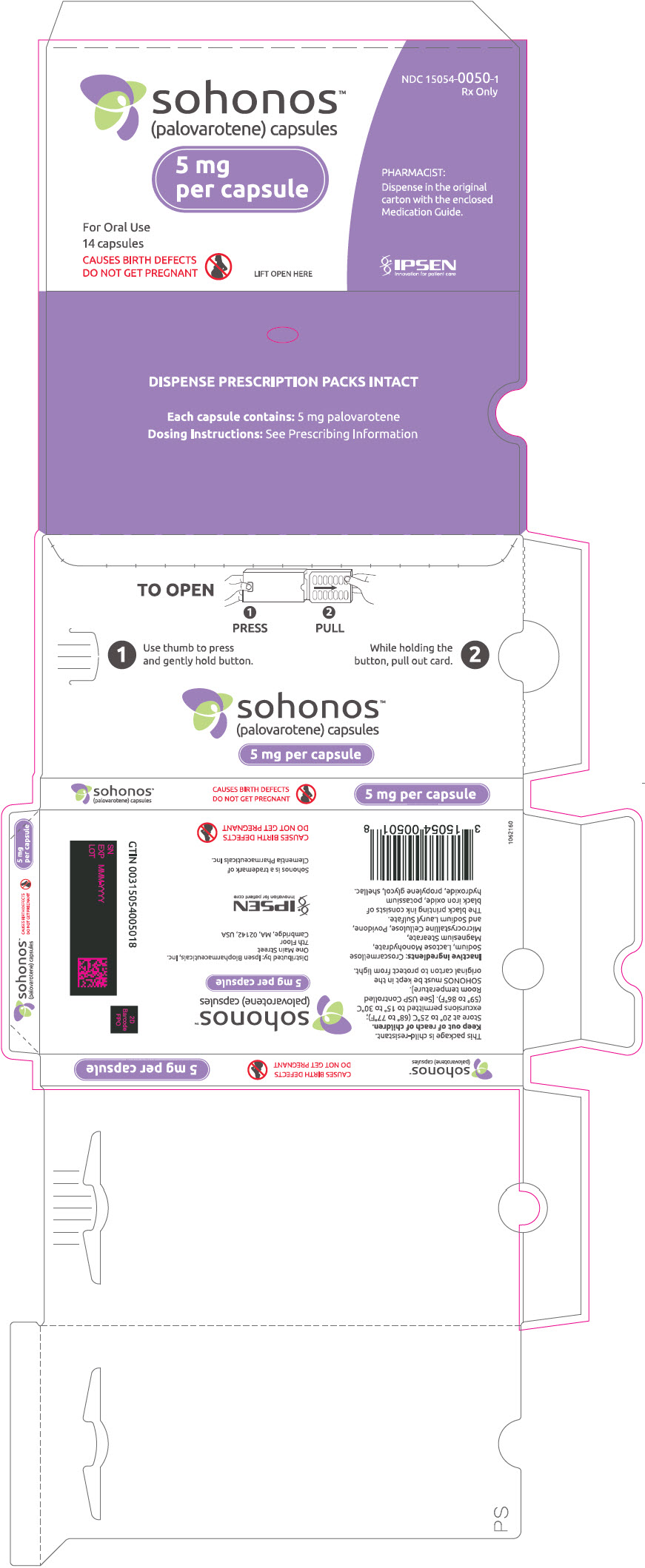
-
PRINCIPAL DISPLAY PANEL - 10 mg Capsule Blister Pack Carton
NDC: 15054-0100-1
Rx Onlysohonos™
(palovarotene) capsules10 mg
per capsuleFor Oral Use
14 capsulesCAUSES BIRTH DEFECTS
DO NOT GET PREGNANTLIFT OPEN HERE
PHARMACIST:
Dispense in the original
carton with the enclosed
Medication Guide.IPSEN
Innovation for patient care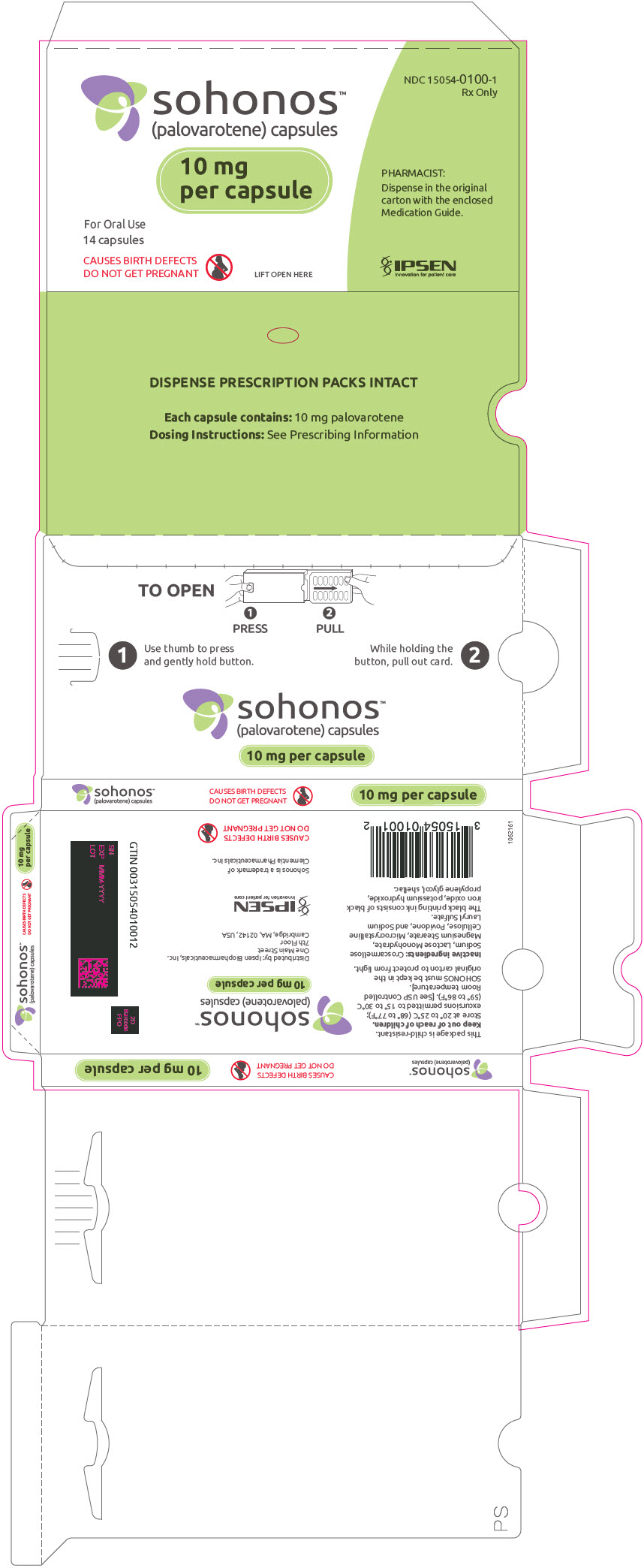
-
INGREDIENTS AND APPEARANCE
SOHONOS
palovarotene capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 15054-0010 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength palovarotene (UNII: 28K6I5M16G) (palovarotene - UNII:28K6I5M16G) palovarotene 1 mg Inactive Ingredients Ingredient Name Strength croscarmellose sodium (UNII: M28OL1HH48) lactose monohydrate (UNII: EWQ57Q8I5X) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) sodium lauryl sulfate (UNII: 368GB5141J) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) Titanium Dioxide (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) propylene glycol (UNII: 6DC9Q167V3) shellac (UNII: 46N107B71O) Product Characteristics Color WHITE (Opaque white) Score no score Shape CAPSULE Size 23mm Flavor Imprint Code PVO;1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 15054-0010-1 1 in 1 CARTON 08/16/2023 1 14 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215559 08/16/2023 SOHONOS
palovarotene capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 15054-0015 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength palovarotene (UNII: 28K6I5M16G) (palovarotene - UNII:28K6I5M16G) palovarotene 1.5 mg Inactive Ingredients Ingredient Name Strength croscarmellose sodium (UNII: M28OL1HH48) lactose monohydrate (UNII: EWQ57Q8I5X) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) sodium lauryl sulfate (UNII: 368GB5141J) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) Titanium Dioxide (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) propylene glycol (UNII: 6DC9Q167V3) shellac (UNII: 46N107B71O) Product Characteristics Color WHITE (Opaque white) Score no score Shape CAPSULE Size 23mm Flavor Imprint Code PVO;1;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 15054-0015-1 1 in 1 CARTON 08/16/2023 1 14 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215559 08/16/2023 SOHONOS
palovarotene capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 15054-0025 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength palovarotene (UNII: 28K6I5M16G) (palovarotene - UNII:28K6I5M16G) palovarotene 2.5 mg Inactive Ingredients Ingredient Name Strength croscarmellose sodium (UNII: M28OL1HH48) lactose monohydrate (UNII: EWQ57Q8I5X) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) sodium lauryl sulfate (UNII: 368GB5141J) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) Titanium Dioxide (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) propylene glycol (UNII: 6DC9Q167V3) shellac (UNII: 46N107B71O) Product Characteristics Color WHITE (Opaque white) Score no score Shape CAPSULE Size 23mm Flavor Imprint Code PVO;2;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 15054-0025-1 1 in 1 CARTON 08/16/2023 1 14 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215559 08/16/2023 SOHONOS
palovarotene capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 15054-0050 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength palovarotene (UNII: 28K6I5M16G) (palovarotene - UNII:28K6I5M16G) palovarotene 5 mg Inactive Ingredients Ingredient Name Strength croscarmellose sodium (UNII: M28OL1HH48) lactose monohydrate (UNII: EWQ57Q8I5X) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) sodium lauryl sulfate (UNII: 368GB5141J) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) Titanium Dioxide (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) propylene glycol (UNII: 6DC9Q167V3) shellac (UNII: 46N107B71O) Product Characteristics Color WHITE (Opaque white) Score no score Shape CAPSULE Size 23mm Flavor Imprint Code PVO;5 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 15054-0050-1 1 in 1 CARTON 08/16/2023 1 14 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215559 08/16/2023 SOHONOS
palovarotene capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 15054-0100 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength palovarotene (UNII: 28K6I5M16G) (palovarotene - UNII:28K6I5M16G) palovarotene 10 mg Inactive Ingredients Ingredient Name Strength croscarmellose sodium (UNII: M28OL1HH48) lactose monohydrate (UNII: EWQ57Q8I5X) magnesium stearate (UNII: 70097M6I30) microcrystalline cellulose (UNII: OP1R32D61U) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) sodium lauryl sulfate (UNII: 368GB5141J) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) Titanium Dioxide (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) propylene glycol (UNII: 6DC9Q167V3) shellac (UNII: 46N107B71O) Product Characteristics Color WHITE (Opaque white) Score no score Shape CAPSULE Size 23mm Flavor Imprint Code PVO;10 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 15054-0100-1 1 in 1 CARTON 08/16/2023 1 14 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA215559 08/16/2023 Labeler - Ipsen Biopharmaceuticals, Inc. (118461578)
Trademark Results [SOHONOS]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SOHONOS 97380247 not registered Live/Pending |
Clementia Pharmaceuticals Inc. 2022-04-25 |
 SOHONOS 90343280 not registered Live/Pending |
Clementia Pharmaceuticals Inc. 2020-11-25 |
 SOHONOS 88493547 not registered Live/Pending |
Clementia Pharmaceuticals, Inc. 2019-06-28 |
 SOHONOS 88185431 not registered Live/Pending |
Clementia Pharmaceuticals, Inc. 2018-11-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.