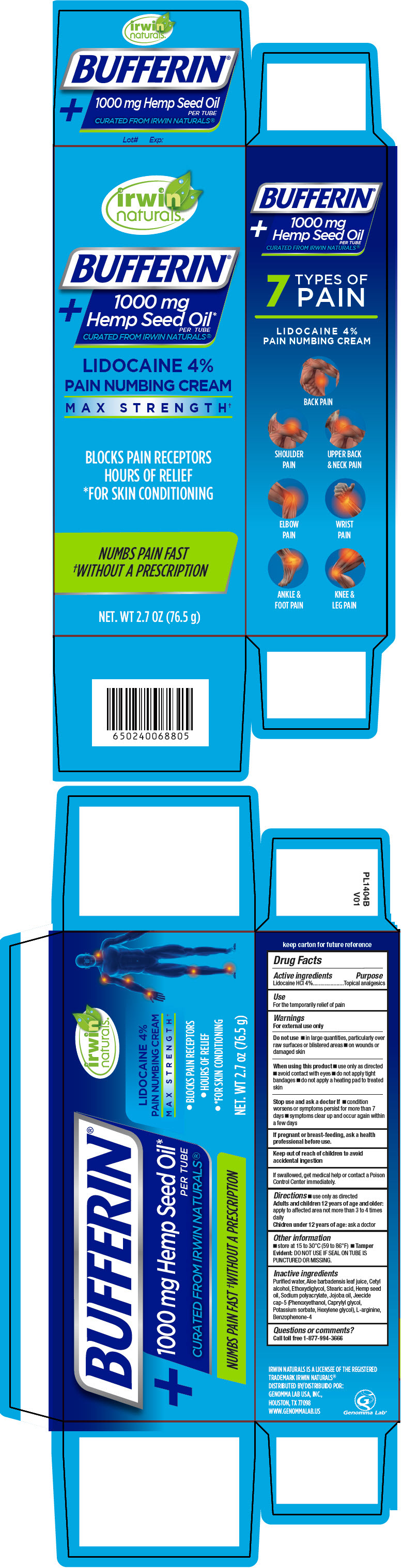
Bufferin® Lidocaine Pain Numbing Cream
Bufferin Lidocaine Pain Numbing by
Drug Labeling and Warnings
Bufferin Lidocaine Pain Numbing by is a Otc medication manufactured, distributed, or labeled by Genomma Lab USA. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BUFFERIN LIDOCAINE PAIN NUMBING- lidocaine hydrochloride cream
Genomma Lab USA
----------
Bufferin® Lidocaine Pain Numbing Cream
Warnings
For external use only
Do not use
- in large quantities, particularly over raw surfaces or blistered areas
- on wounds or damaged skin
When using this product
- use only as directed
- avoid contact with eyes
- do not apply tight bandages
- do not apply a heating pad to treated skin
Directions
- use only as directed
Other information
- store at 15 to 30"C (59 to 86"F)
- Tamper Evident: DO NOT USE IF SEAL ON TUBE IS PUNCTURED OR MISSING.
| BUFFERIN LIDOCAINE PAIN NUMBING
lidocaine hydrochloride cream |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Genomma Lab USA (832323534) |
Revised: 1/2025
Document Id: 29a0240b-77e8-4daf-ac9b-e318fc42bed8
Set id: 67fac18d-62e4-4ae4-9c7f-a9427df32afb
Version: 2
Effective Time: 20250129
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.