Ventiva Lubricant Eye Drops by Singular Dreamer dba True Marker
Ventiva Lubricant Eye Drops by
Drug Labeling and Warnings
Ventiva Lubricant Eye Drops by is a Otc medication manufactured, distributed, or labeled by Singular Dreamer dba True Marker. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
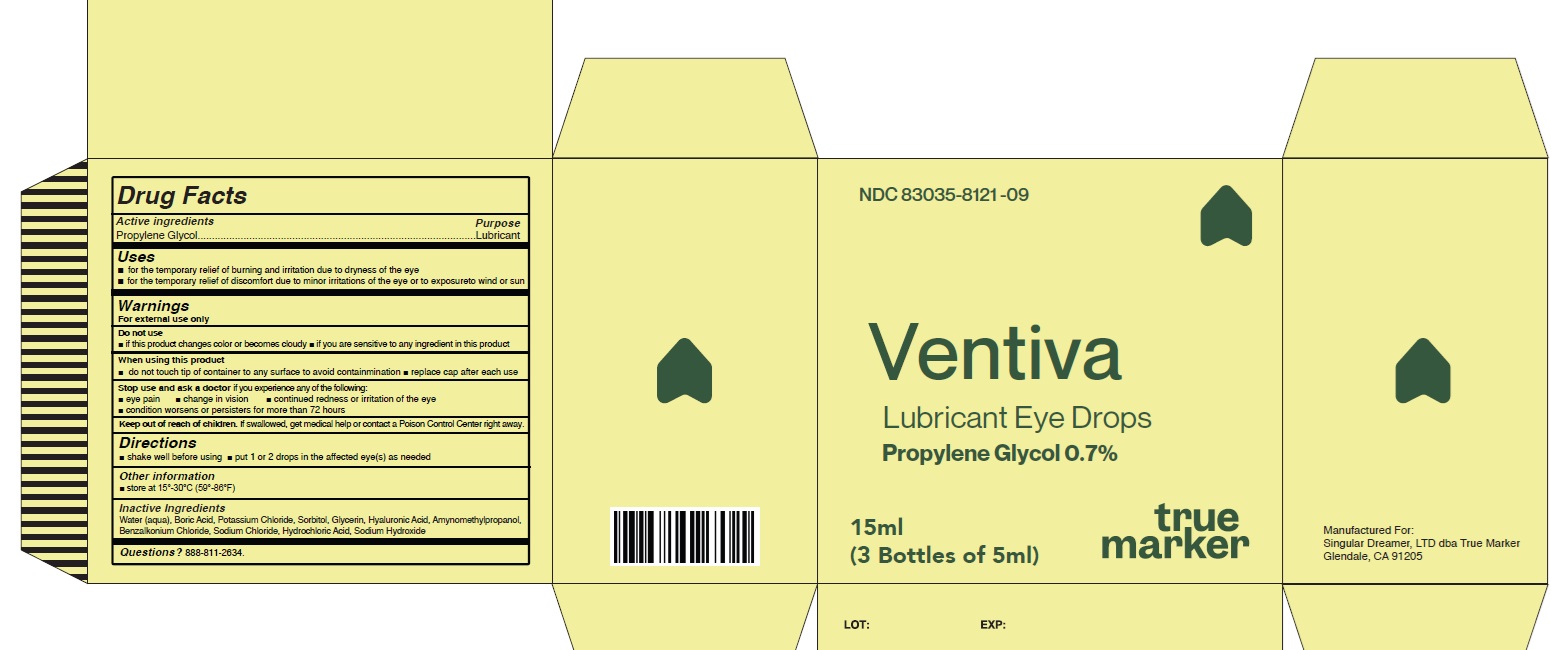
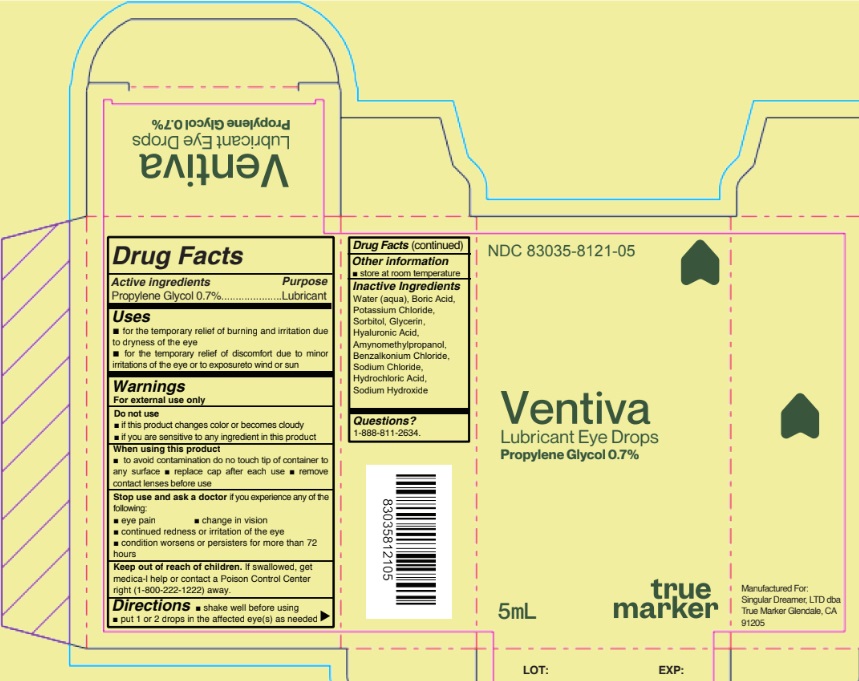
VENTIVA LUBRICANT EYE DROPS- propylene glycol liquid
Singular Dreamer dba True Marker
----------
Uses
For the temporary relief of burning and irritation due to dryness of the eye, for the temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
Warnings
For external use only
Do not use
- if this product changes color or becomes cloudy
- if you are sensitive to any ingredient in this product
When using this product
- do not touch tip of container to any surface to avoid contamination
- replace cap after each use
Stop use and ask a doctorif you experience any of the
following:
- eye pain
- change in vision
- continued redness or irritation of the eye
- condition worsens or persists for more than 72 hours
Keep out of reach of children. If swallowed, get
medical help or contact a Poison Control Center right
away.
| VENTIVA LUBRICANT EYE DROPS
propylene glycol liquid |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Singular Dreamer dba True Marker (129504103) |
| Registrant - Singular Dreamer dba True Marker (129504103) |
Revised: 11/2024
Document Id: 2733bf99-1242-13f1-e063-6394a90ac64d
Set id: 6828dd62-f75d-4ea0-8e6c-a7bf00780093
Version: 6
Effective Time: 20241118