COUGH MUCUS RELIEF DM- dextromethorphan hbr and guaifenesin tablet
Cough Mucus Relief DM by
Drug Labeling and Warnings
Cough Mucus Relief DM by is a Otc medication manufactured, distributed, or labeled by Strive Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
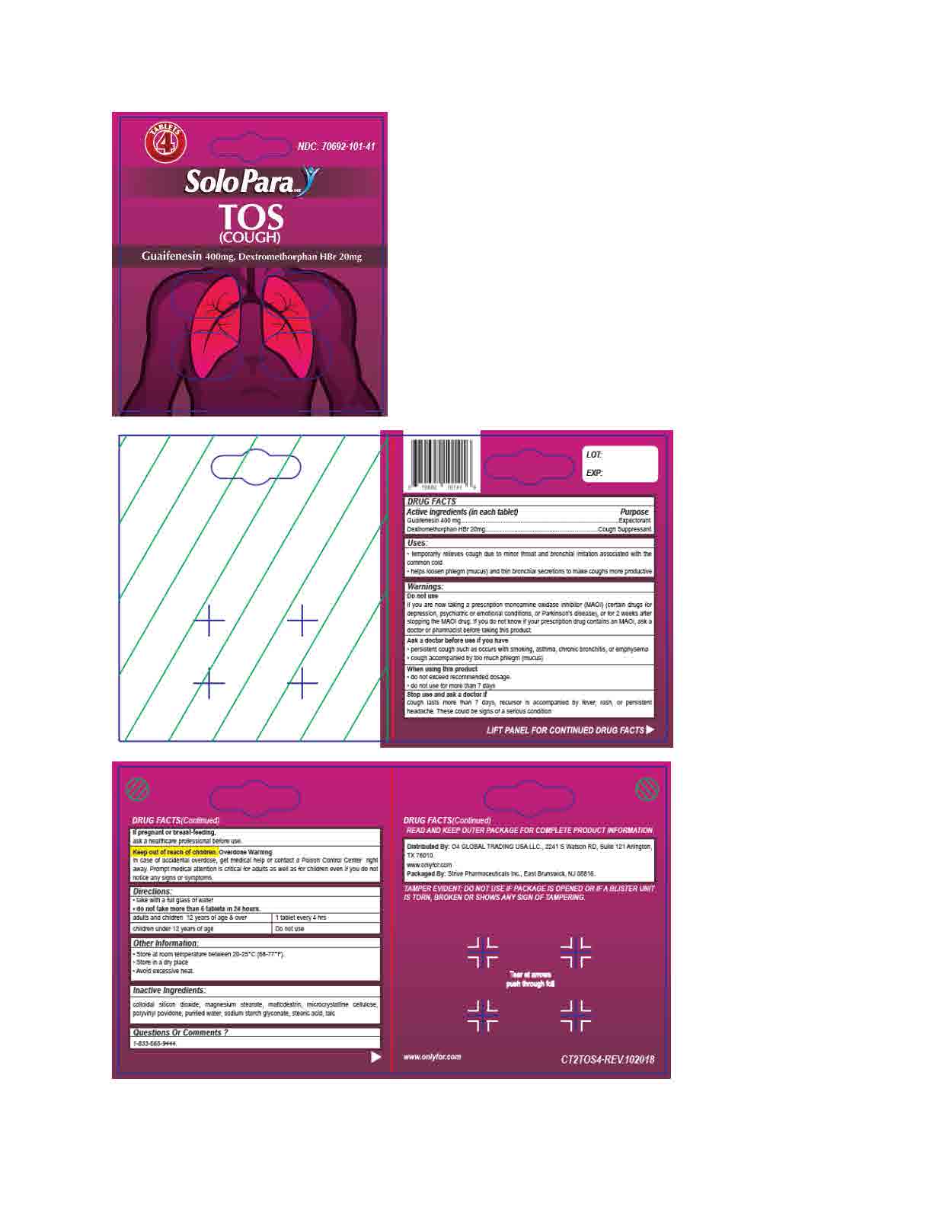
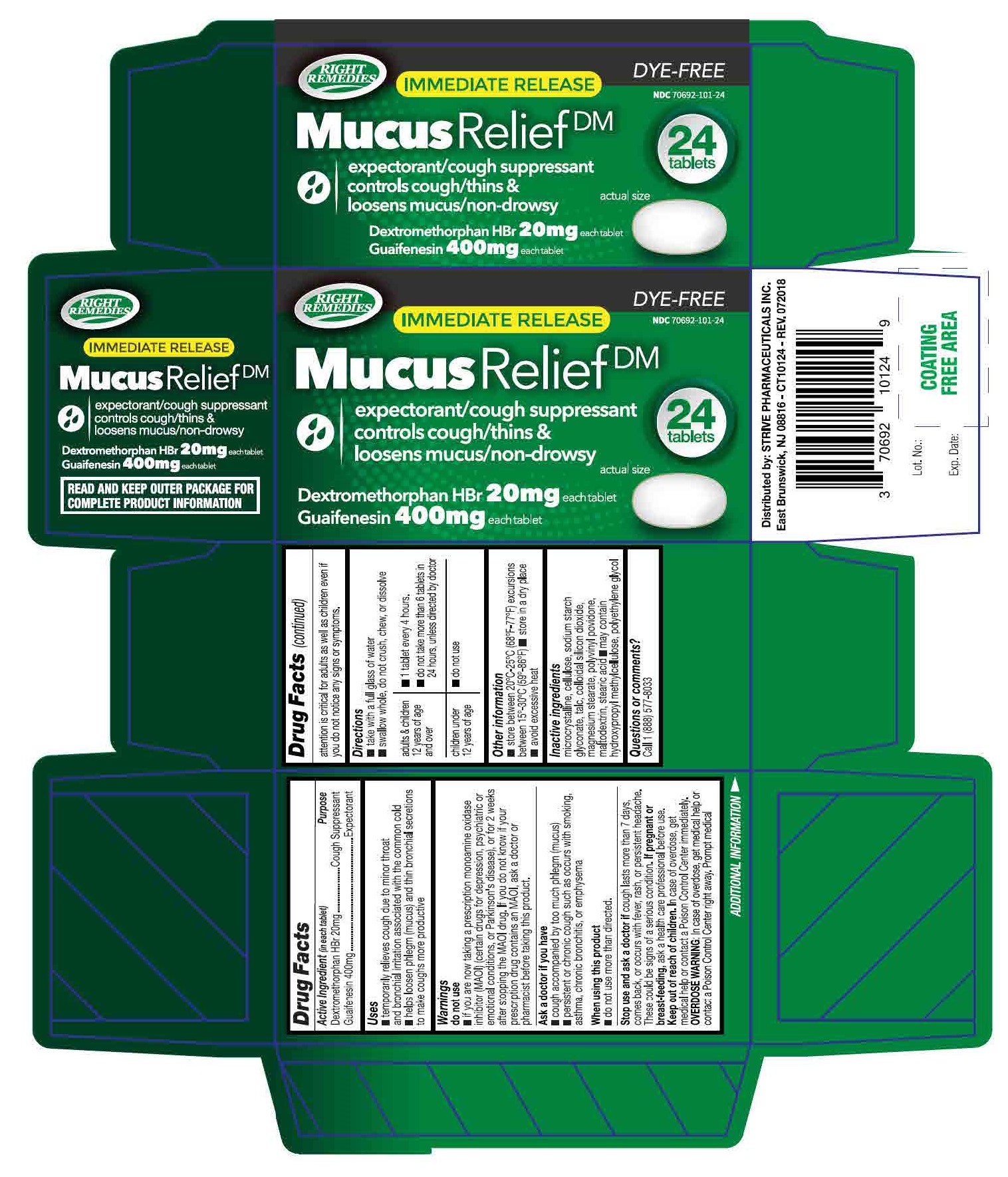
- ACTIVE INGREDIENT
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- INDICATIONS & USAGE
-
WARNINGS
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
* persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis,or emphysema
* cough accompanied by too much phlegm (mucus)
When using this product do not use more than directed
Stop use and ask a doctor if cough lasts more than 7 days, comes back, or occurs with fever, rash, or persistent headache. These could be signs of a serious condition.
If pregnant or breast-feeding, ask a health professional before use.
Keep out of reach of children. In case of accidental overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- OTHER SAFETY INFORMATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COUGH MUCUS RELIEF DM
dextromethorphan hbr and guaifenesin tabletProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70692-101 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg Inactive Ingredients Ingredient Name Strength HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A CORN (UNII: AG9B65PV6B) TALC (UNII: 7SEV7J4R1U) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) MALTODEXTRIN (UNII: 7CVR7L4A2D) STEARIC ACID (UNII: 4ELV7Z65AP) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) Product Characteristics Color white Score 2 pieces Shape OVAL Size 16mm Flavor Imprint Code S55 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70692-101-24 24 in 1 BOTTLE; Type 0: Not a Combination Product 02/15/2018 2 NDC: 70692-101-41 4 in 1 PACKAGE; Type 0: Not a Combination Product 12/20/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 02/15/2018 Labeler - Strive Pharmaceuticals Inc. (080028013)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.