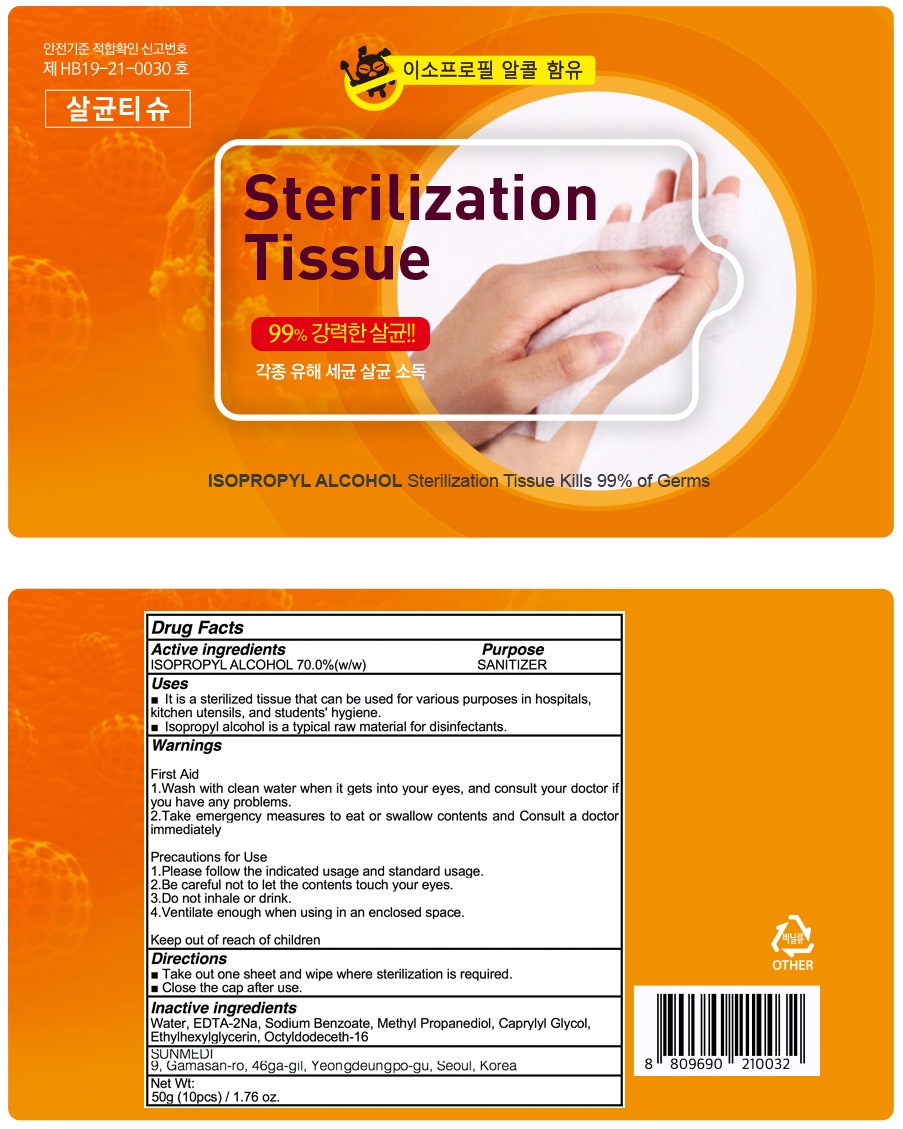
Gyun e zero sterilization tissue by Sunmedical
Gyun e zero sterilization tissue by
Drug Labeling and Warnings
Gyun e zero sterilization tissue by is a Otc medication manufactured, distributed, or labeled by Sunmedical. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GYUN E ZERO STERILIZATION TISSUE- alcohol liquid
Sunmedical
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
INACTIVE INGREDIENT
Inactive ingredients:
Water, EDTA-2Na, Sodium Benzoate, Methyl Propanediol, Caprylyl Glycol, Ethylhexylglycerin, Octyldodeceth-16
Warnings
Warnings:
First Aid
1.Wash with clean water when it gets into your eyes, and consult your doctor if you have any problems.
2.Take emergency measures to eat or swallow contents and Consult a doctor immediately
Precautions for Use
1.Please follow the indicated usage and standard usage.
2.Be careful not to let the contents touch your eyes.
3.Do not inhale or drink.
4.Ventilate enough when using in an enclosed space.
Uses
Uses:
■ It is a sterilized tissue that can be used for various purposes in hospitals, kitchen utensils, and students' hygiene.
■ Isopropyl alcohol is a typical raw material for disinfectants.
| GYUN E ZERO STERILIZATION TISSUE
alcohol liquid |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Sunmedical (688954380) |
| Registrant - Sunmedical (688954380) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sunmedical | 688954380 | manufacture(75578-010) | |