SEI BELLA CC BRIGHTENING CREME MEDIUM- octinoxate 7.5%, titanium dioxide 5%, zinc oxide 1% lotion
Sei Bella CC Brightening Creme by
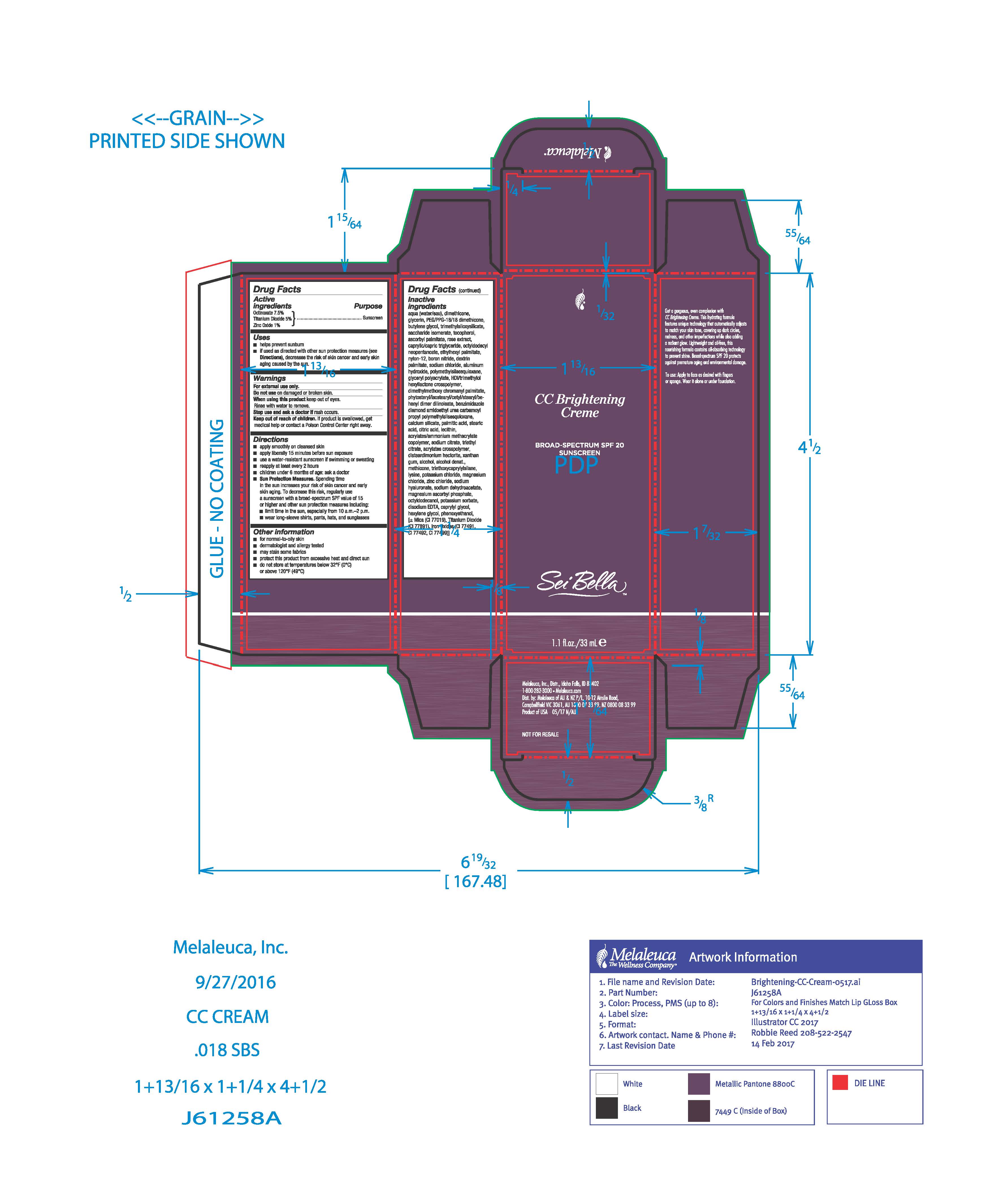
Drug Labeling and Warnings
Sei Bella CC Brightening Creme by is a Otc medication manufactured, distributed, or labeled by Melaleuca Inc., Mana. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Use
- Warnings
- WHEN USING
- STOP USE
- Keep out of reach of children.
-
Directions
■ apply after cleanser, toner, and foundation primer. Blend outward with Sei Bella foundation brush or fingers.
■ apply liberally 15 minutes before sun exposure
■ use a water-resistant sunscreen if swimming or sweating
■ reapply at least every 2 hours
■ children under 6 months: ask a doctor
■ Sun Protection Measures. Spending time in the sun increases you risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad-spectrum SPF value of 15 or higher and other sun protection measures including:
■ limit time in the sun, especially from 10 a.m.-2 p.m.
■ wear long-sleeve shirts, pants, hats, and sunglasses
-
Inactive ingredients
Aqua/Water/Eau, Dimethicone, Glycerin, PEG/PPG-18/18 Dimethicone, Butylene Glycol, Trimethylsiloxysilicate, Saccharide Isomerate, Tocopherol, Ascorbyl Palmitate, Rose Extract, Caprylic/Capric Triglyceride, Octyldodecyl Neopentanoate , Ethylhexyl Palmitate, Nylon-12, Boron Nitride, Dextrin Palmitate, Sodium Chloride, Aluminum Hydroxide, Polymethylsilsesquioxane, Glyceryl Polyacrylate, HDI/Trimethylol Hexyllactone Crosspolymer, Dimethylmethoxy Chromanyl Palmitate, Phytosteryl/Isostearyl/Cetyl/Stearyl/Behenyl Dimer Dilinoleate, Benzimidazole Diamond Amidoethyl Urea Carbamoyl Propyl Polymethylsilsesquioxane, Calcium Silicate, Palmitic Acid, Stearic Acid, Citric Acid , Lecithin, Acrylates/Ammonium Methacrylate Copolymer, Sodium Citrate, Triethyl Citrate , Acrylates Crosspolymer, Disteardimonium Hectorite, Xanthan Gum, Alcohol, Alcohol Denat., Methicone, Triethoxycaprylylsilane, Lysine, Potassium Chloride, Magnesium Chloride, Zinc Chloride, Sodium Hyaluronate, Sodium Dehydroacetate, Magnesium Ascorbyl Phosphate, Octyldodecanol, Potassium Sorbate, Disodium EDTA, Caprylyl Glycol, Hexylene Glycol, Phenoxyethanol
May Contain (+/-)
Mica, Titanium Dioxide (CI 77891), Iron Oxides (CI 77491, CI 77492, CI 77499) - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SEI BELLA CC BRIGHTENING CREME MEDIUM
octinoxate 7.5%, titanium dioxide 5%, zinc oxide 1% lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 54473-281 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.65 g in 33 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2.475 g in 33 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.3267 g in 33 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ROSA CENTIFOLIA FLOWER OIL (UNII: H32V31VMWY) NYLON-12 (UNII: 446U8J075B) HEXAMETHYLENE DIISOCYANATE/TRIMETHYLOL HEXYLLACTONE CROSSPOLYMER (UNII: WB5K9Y35Y9) DIMETHYLMETHOXY CHROMANYL PALMITATE (UNII: 5G222ZDK7U) CALCIUM SILICATE (UNII: S4255P4G5M) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) AMMONIUM METHACRYLATE (UNII: J2243103QO) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) MAGNESIUM CHLORIDE (UNII: 02F3473H9O) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) DIMETHICONE (UNII: 92RU3N3Y1O) ETHYLHEXYL PALMITATE (UNII: 2865993309) SODIUM CHLORIDE (UNII: 451W47IQ8X) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) XANTHAN GUM (UNII: TTV12P4NEE) OCTYLDODECANOL (UNII: 461N1O614Y) BORON NITRIDE (UNII: 2U4T60A6YD) SODIUM DEHYDROACETATE (UNII: 8W46YN971G) PHENOXYETHANOL (UNII: HIE492ZZ3T) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) TRIMETHYLSILOXYSILICATE (M/Q 0.6-0.8) (UNII: 5041RX63GN) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) PHYTOSTERYL/ISOSTEARYL/CETYL/STEARYL/BEHENYL DIMER DILINOLEATE (UNII: 8N725H4EFN) PALMITIC ACID (UNII: 2V16EO95H1) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM CITRATE (UNII: 1Q73Q2JULR) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) DISTEARDIMONIUM HECTORITE (UNII: X687XDK09L) ALCOHOL (UNII: 3K9958V90M) METHICONE (20 CST) (UNII: 6777U11MKT) MAGNESIUM ASCORBYL PHOSPHATE (UNII: 0R822556M5) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SACCHARIDE ISOMERATE (UNII: W8K377W98I) TOCOPHEROL (UNII: R0ZB2556P8) ASCORBYL PALMITATE (UNII: QN83US2B0N) CAPRYLIC/CAPRIC/LINOLEIC TRIGLYCERIDE (UNII: U73D397055) ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) BENZIMIDAZOLE (UNII: E24GX49LD8) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DEXTRIN PALMITATE (CORN; 20000 MW) (UNII: 89B2BSF9I3) POTASSIUM CHLORIDE (UNII: 660YQ98I10) ZINC CHLORIDE (UNII: 86Q357L16B) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54473-281-01 1 in 1 BOX 01/01/2018 1 NDC: 54473-281-30 33 mL in 1 BOTTLE, DISPENSING; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/01/2017 Labeler - Melaleuca Inc. (139760102) Registrant - Melaleuca Inc. (139760102) Establishment Name Address ID/FEI Business Operations Mana 078870292 manufacture(54473-281)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.