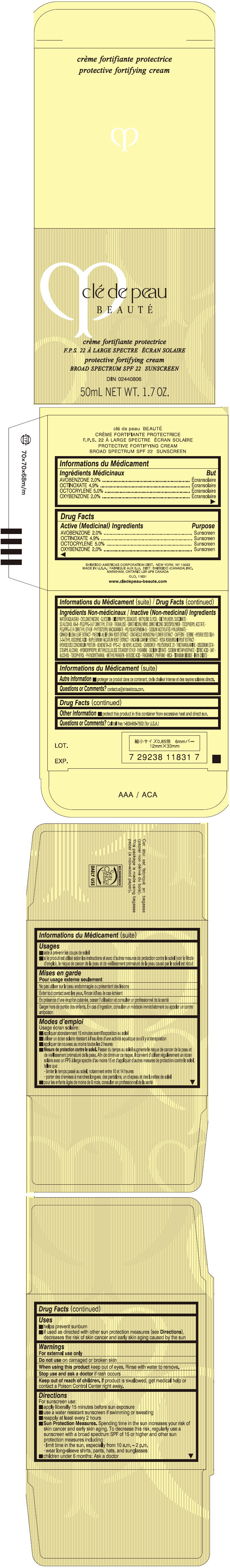
CLE DE PEAU BEAUTE PROTECTIVE FORTIFYING- avobenzone, octinoxate, octocrylene, and oxybenzone cream
CLE DE PEAU BEAUTE PROTECTIVE FORTIFYING by
Drug Labeling and Warnings
CLE DE PEAU BEAUTE PROTECTIVE FORTIFYING by is a Otc medication manufactured, distributed, or labeled by SHISEIDO AMERICAS CORPORATION, SHISEIDO AMERICA INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
-
Inactive Ingredients
WATER (AQUA/EAU)∙CYCLOMETHICONE∙GLYCERIN∙DIISOPROPYL SEBACATE∙BUTYLENE GLYCOL∙DIETHYLHEXYL SUCCINATE∙SD ALCOHOL 40-B∙PEG/PPG-14/7 DIMETHYL ETHER∙TREHALOSE∙DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER∙TOCOPHERYL ACETATE∙PEG/PPG-17/4 DIMETHYL ETHER∙PHYTOSTERYL MACADAMIATE∙POLYQUATERNIUM-51∙SODIUM ACETYLATED HYALURONATE∙GINKGO BILOBA LEAF EXTRACT∙PAEONIA ALBIFLORA ROOT EXTRACT∙CRATAEGUS MONOGYNA FLOWER EXTRACT∙CAFFEINE∙SERINE∙HYDROLYZED SILK∙2-O-ETHYL ASCORBIC ACID∙BUPLEURUM FALCATUM ROOT EXTRACT∙UNCARIA GAMBIR EXTRACT∙ROSA ROXBURGHII FRUIT EXTRACT∙HYDROLYZED CONCHIOLIN PROTEIN∙BEHENETH-20∙PPG-17∙BEHENYL ALCOHOL∙CARBOMER∙POLYSORBATE 20∙TRIETHANOLAMINE∙DISODIUM EDTA∙STEARYL ALCOHOL∙HYDROXYPROPYL METHYLCELLULOSE STEAROXY ETHER∙THEANINE∙SODIUM CITRATE∙SODIUM METAPHOSPHATE∙CITRIC ACID∙BHT∙ALCOHOL∙TOCOPHEROL∙PHENOXYETHANOL∙METHYLPARABEN∙BENZOIC ACID∙FRAGRANCE (PARFUM)∙MICA∙TITANIUM DIOXIDE∙IRON OXIDES∙
- Other information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 50 mL Jar Carton
-
INGREDIENTS AND APPEARANCE
CLE DE PEAU BEAUTE PROTECTIVE FORTIFYING
avobenzone, octinoxate, octocrylene, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 58411-258 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1020 mg in 50 mL OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 2499 mg in 50 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 2550 mg in 50 mL OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 1020 mg in 50 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE (UNII: NMQ347994Z) GLYCERIN (UNII: PDC6A3C0OX) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) DIETHYLHEXYL SUCCINATE (UNII: 69W9UMG3P8) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) TREHALOSE (UNII: B8WCK70T7I) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) PEG/PPG-17/4 DIMETHYL ETHER (UNII: 4ET18WJG5K) SODIUM ACETYLATED HYALURONATE (UNII: WN66R7GL93) GINKGO (UNII: 19FUJ2C58T) PAEONIA LACTIFLORA ROOT (UNII: 3Z3866YW6P) CRATAEGUS MONOGYNA FLOWER (UNII: NT52AMP29J) CAFFEINE (UNII: 3G6A5W338E) SERINE (UNII: 452VLY9402) 2-O-ETHYL ASCORBIC ACID (UNII: 801M14RK9K) BUPLEURUM FALCATUM ROOT (UNII: X04E310LUY) ROSA ROXBURGHII FRUIT (UNII: CVT1AA87FF) BEHENETH-20 (UNII: BJ4GP2IFLN) PPG-17 (UNII: OV0Q322E0U) DOCOSANOL (UNII: 9G1OE216XY) CARBOXYPOLYMETHYLENE (UNII: 0A5MM307FC) POLYSORBATE 20 (UNII: 7T1F30V5YH) TROLAMINE (UNII: 9O3K93S3TK) EDETATE DISODIUM (UNII: 7FLD91C86K) STEARYL ALCOHOL (UNII: 2KR89I4H1Y) THEANINE (UNII: 8021PR16QO) SODIUM CITRATE (UNII: 1Q73Q2JULR) SODIUM POLYMETAPHOSPHATE (UNII: P1BM4ZH95L) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) ALCOHOL (UNII: 3K9958V90M) .ALPHA.-TOCOPHEROL (UNII: H4N855PNZ1) PHENOXYETHANOL (UNII: HIE492ZZ3T) METHYLPARABEN (UNII: A2I8C7HI9T) BENZOIC ACID (UNII: 8SKN0B0MIM) MICA (UNII: V8A1AW0880) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERRIC OXIDE RED (UNII: 1K09F3G675) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58411-258-50 1 in 1 CARTON 02/01/2016 1 50 mL in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 02/01/2016 Labeler - SHISEIDO AMERICAS CORPORATION (193691821) Establishment Name Address ID/FEI Business Operations SHISEIDO AMERICA INC. 782677132 manufacture(58411-258) , analysis(58411-258)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.