CHILDRENS CHEWABLE- acetaminophen tablet, chewable
Childrens Chewable by
Drug Labeling and Warnings
Childrens Chewable by is a Otc medication manufactured, distributed, or labeled by Salado Sales, Inc., LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each chewable tablet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if your child takes:
- more than 5 doses in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Sore throat warning: If sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if your child is allergic to acetaminophen or any of the inactive ingredients in this product
-
Directions
- this product does not contain directions or complete warnings for adult use
- do not give more than directed
- find the right dose on chart below. If possible, use weight to dose; otherwise, use age.
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- repeat dose every 4 hours while symptoms last
- do not give more than 5 times in 24 hours
- do not give for more than 5 days unless directed by a doctor
Weight (lb) Age (yr) Dose (chewable tablets)* Under 24
Under 2 Ask a doctor 24-35 2-3 1 tablet 36-47 4-5 1 1/2 tablet 48-59 6-8 2 tablets 60-71 9-10 2 1/2 tablets 72-95 11 3 tablets *or as directed by a doctor
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
CVP®
HEALTHCOMPARE TO
CHILDREN'S TYLENOL®
ACTIVE INGREDIENT†AGES 2-11
CHILDREN'S
CHEWABLEACETAMINOPHEN 160 mg
CHEWABLE TABLETS
PAIN RELIEVER/FEVER REDUCER
IBUPROFEN FREE/ASPIRIN FREE12 CHEWABLE
TABLETSChew or crush tablets
completely before swallowing.TAMPER EVIDENT: DO NOT USE IF
IMPRINTED SAFETY SEAL UNDER CAP
IS BROKEN OR MISSING†This product is not manufactured or distributed by Johnson & Johnson
Corporation, owner of the registered trademark Children's Tylenol®.
50844 REV1018A45002Distributed by Consumer Value Products, Inc.
P.O. Box 6115, Temple, Texas 76502
CVPproducts.com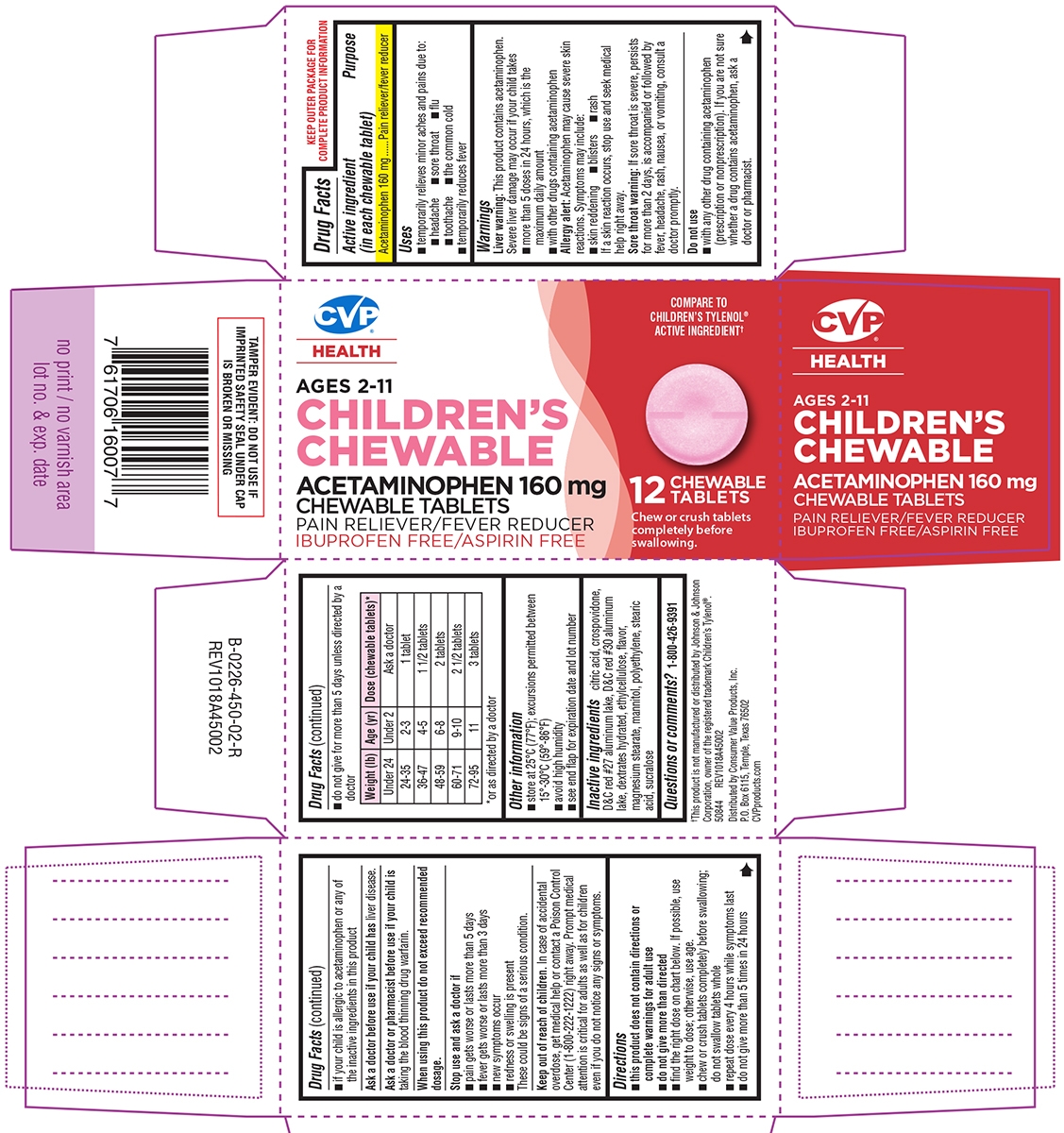
CVP 44-450
-
INGREDIENTS AND APPEARANCE
CHILDRENS CHEWABLE
acetaminophen tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 57243-450 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 160 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) D&C RED NO. 27 ALUMINUM LAKE (UNII: ZK64F7XSTX) D&C RED NO. 30 (UNII: 2S42T2808B) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) ETHYLCELLULOSE, UNSPECIFIED (UNII: 7Z8S9VYZ4B) STEARIC ACID (UNII: 4ELV7Z65AP) SUCRALOSE (UNII: 96K6UQ3ZD4) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) HIGH DENSITY POLYETHYLENE (UNII: UG00KM4WR7) Product Characteristics Color PINK Score 2 pieces Shape ROUND Size 16mm Flavor BUBBLE GUM Imprint Code 44;450 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57243-450-02 1 in 1 CARTON 02/25/2005 1 12 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part343 02/25/2005 Labeler - Salado Sales, Inc. (009830555) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(57243-450) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(57243-450) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(57243-450) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(57243-450) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(57243-450)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.