TALICIA- omeprazole magnesium, amoxicillin and rifabutin capsule, delayed release
Talicia by
Drug Labeling and Warnings
Talicia by is a Prescription medication manufactured, distributed, or labeled by RedHill Biopharma Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use TALICIA® safely and effectively. See full prescribing information for TALICIA.
TALICIA (omeprazole magnesium, amoxicillin and rifabutin) delayed-release capsules, for oral use
Initial U.S. Approval: 2019INDICATIONS AND USAGE
TALICIA is a three-drug combination of omeprazole, a proton pump inhibitor, amoxicillin, a penicillin-class antibacterial, and rifabutin, a rifamycin antibacterial, indicated for the treatment of Helicobacter pylori infection in adults. (1)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of TALICIA and other antibacterial drugs, TALICIA should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Delayed Release Capsule: Omeprazole 10 mg, (equivalent to 10.3 mg of omeprazole magnesium) amoxicillin 250 mg and rifabutin 12.5 mg. (3)
CONTRAINDICATIONS
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: Serious and occasionally fatal reactions (e.g., anaphylaxis) have been reported with components of TALICIA. If hypersensitivity reactions occur, discontinue TALICIA and institute immediate therapy (e.g., anaphylaxis management). (5.1)
- Clostridioides difficile-Associated Diarrhea (CDAD): Evaluate if diarrhea occurs. (5.2)
- Reduction in the Efficacy of Hormonal Contraceptives: Additional non-hormonal highly effective methods of contraception should be used while taking TALICIA. (5.3)
- Acute Interstitial Nephritis (AIN): Observed in patients taking (Proton Pump Inhibitors (PPIs) and penicillins. Discontinue TALICIA if AIN develops. (5.4)
- Cutaneous and Systemic Lupus Erythematosus: Mostly cutaneous; new onset or exacerbation of existing disease; discontinue TALICIA and evaluate. (5.5)
ADVERSE REACTIONS
Most common adverse reactions (≥1%) were diarrhea, headache, nausea, abdominal pain, chromaturia, rash, dyspepsia, oropharyngeal pain, vomiting, and vulvovaginal candidiasis. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact RedHill Biopharma Inc. at 1-833-ADRHILL (1-833-237-4455) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Helicobacter pylori Infection
1.2 Usage
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity Reactions
4.2 Rilpivirine-containing Products
4.3 Delavirdine
4.4 Voriconazole
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
5.2 Clostridioides difficile-Associated Diarrhea
5.3 Reduced Efficacy of Hormonal Contraceptives
5.4 Acute Interstitial Nephritis
5.5 Risk of Adverse Reactions or Loss of Efficacy Due to Drug Interactions
5.6 Cutaneous and Systemic Lupus Erythematosus
5.7 Rash in Patients with Mononucleosis
5.8 Uveitis
5.9 Interactions with Diagnostic Investigations for Neuroendocrine Tumors
5.10 Development of Drug-Resistant Bacteria
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience with TALICIA
6.2 Other Important Adverse Reactions from the Labeling of the Individual Components of TALICIA
6.3 Post-Marketing Experience with Components of TALICIA
7 DRUG INTERACTIONS
7.1 Interactions with Other Drugs and Diagnostics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
12.4 Microbiology
12.5 Pharmacogenomics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Helicobacter pylori Infection
TALICIA is indicated for the treatment of Helicobacter pylori infection in adults [see Clinical Studies (14)].
1.2 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of TALICIA and other antibacterial drugs, TALICIA should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION
Administer four (4) TALICIA capsules every 8 hours for 14 days with food. Instruct patients to swallow the TALICIA capsules whole, with a full glass of water (8 ounces). Each dose (4 capsules) of TALICIA includes rifabutin 50 mg, amoxicillin 1,000 mg and omeprazole 40 mg. Do not crush or chew TALICIA capsules. Do not take TALICIA with alcohol.
If a dose is missed, patients should continue the normal dosing schedule until the medication is completed. Do not take two doses at one time to make up for a missed dose.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity Reactions
TALICIA is contraindicated in patients with known hypersensitivity to the components of TALICIA: amoxicillin [or other β-lactam antibacterial drugs (e.g., penicillins and cephalosporins)], omeprazole (or other benzimidazoles [e.g. proton pump inhibitors (PPIs) and anthelmintics]), rifabutin (or any other rifamycins), or to any other component of TALICIA. Hypersensitivity reactions may include anaphylaxis or Stevens Johnson Syndrome, anaphylactic shock, angioedema, bronchospasm, interstitial nephritis, rash and urticaria [see Warnings and Precautions (5.1), Adverse Reactions (6.1)].
4.2 Rilpivirine-containing Products
Proton pump inhibitors (PPIs), including omeprazole (a component of TALICIA), are contraindicated in patients receiving rilpivirine-containing products [see Drug Interactions (7.1)].
4.3 Delavirdine
The use of rifabutin (a component of TALICIA), is contraindicated in patients receiving delavirdine [see Drug Interactions (7.1)].
4.4 Voriconazole
The use of rifabutin (a component of TALICIA), is contraindicated in patients receiving voriconazole [see Drug Interactions (7.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Hypersensitivity Reactions
Serious and fatal hypersensitivity reactions, e.g. anaphylaxis, angioedema, erythema multiforme, Stevens-Johnson syndrome, exfoliative dermatitis, toxic epidermal necrolysis, acute generalized exanthematous pustulosis, hypersensitivity vasculitis, interstitial nephritis, and serum sickness have been reported with the components of TALICIA: omeprazole, amoxicillin and rifabutin.
Signs and symptoms of these reactions may include hypotension, urticaria, angioedema, acute bronchospasm, conjunctivitis, thrombocytopenia, neutropenia or flu-like syndrome (weakness, fatigue, muscle pain, nausea, vomiting, headache, fever, chills, aches, rash, itching, sweats, dizziness, shortness of breath, chest pain, cough, syncope, palpitations).
There have been reports of individuals with a history of penicillin hypersensitivity who have experienced severe reactions when treated with cephalosporins.
Before initiating therapy with TALICIA, inquire about history of hypersensitivity reactions to penicillins, cephalosporins, rifamycins, or PPIs. Discontinue TALICIA and institute immediate therapy, if hypersensitivity reactions occur.
5.2 Clostridioides difficile-Associated Diarrhea
Clostridioides difficile-associated diarrhea (CDAD) has been reported with use of omeprazole, a component of TALICIA and nearly all antibacterial agents, including amoxicillin and rifabutin, which are components of TALICIA and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
CDAD must be considered in all patients who present with diarrhea following proton pump inhibitor and or antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is confirmed, TALICIA should be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial drug treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.3 Reduced Efficacy of Hormonal Contraceptives
TALICIA may reduce the efficacy of hormonal contraceptives. Therefore, an additional non-hormonal highly effective method of contraception should be used while taking TALICIA [see Drug Interactions (7.1)].
5.4 Acute Interstitial Nephritis
Acute interstitial nephritis (AIN) has been observed in patients taking PPIs including omeprazole as well as in patients taking penicillins such as amoxicillin, a component of TALICIA. Acute interstitial nephritis may occur at any point during PPI therapy and is generally attributed to an idiopathic hypersensitivity reaction. Discontinue TALICIA if AIN develops [see Contraindications (4.1)].
5.5 Risk of Adverse Reactions or Loss of Efficacy Due to Drug Interactions
Components of TALICIA have the potential for clinically important drug interactions [see Contraindications (4) and Drug Interactions (7)].
Avoid concomitant use of TALICIA with other CYP2C19 or CYP3A4 inducers (e.g. St. John’s Wort, rifampin) as they can substantially decrease omeprazole concentrations. Avoid concomitant use of TALICIA with CYP2C19 and/or CYP3A4 inhibitors (e.g. fluconazole, itraconazole) as it may significantly increase the plasma concentration of component (s) of TALICIA. Depending on the protease inhibitor, the concomitant use of TALICIA should be avoided (e.g. amprenavir, indinavir) or dose adjustments for a concomitantly administered protease inhibitor(s) may be required. Concomitant use of PPIs with methotrexate (primarily at high dose) may elevate and prolong serum levels of methotrexate and/or its metabolite, possibly leading to methotrexate toxicities. Avoid TALICIA in patients on high-dose methotrexate. Concomitant use of clopidogrel and omeprazole reduces the pharmacological activity of clopidogrel. Avoid TALICIA in patients on clopidogrel. When using TALICIA, consider alternative anti-platelet therapy [see Drug Interactions (7)].
5.6 Cutaneous and Systemic Lupus Erythematosus
Cutaneous lupus erythematosus (CLE) and systemic lupus erythematosus (SLE) have been reported in patients taking PPIs, including omeprazole. These events have occurred as both new onset and an exacerbation of existing autoimmune disease. The majority of PPI-induced lupus erythematosus cases were CLE. If signs or symptoms consistent with CLE or SLE develop in patients receiving TALICIA, discontinue the drug and evaluate as appropriate.
5.7 Rash in Patients with Mononucleosis
A high percentage of patients with mononucleosis who receive amoxicillin develop an erythematous skin rash. Avoid TALICIA in patients with mononucleosis.
5.8 Uveitis
Due to the possible occurrence of uveitis, patients should be carefully monitored when rifabutin, a component of TALICIA, is given in combination with clarithromycin (or other macrolides) and/or fluconazole and related compounds. If uveitis is suspected, refer for an ophthalmologic evaluation and, if considered necessary, suspend treatment with rifabutin [see Adverse Reactions (6.2)].
5.9 Interactions with Diagnostic Investigations for Neuroendocrine Tumors
Serum chromogranin A (CgA) levels increase secondary to drug-induced decreases in gastric acidity. The increased CgA level may cause false positive results in diagnostic investigations for neuroendocrine tumors. Assess CgA levels at least 14 days after TALICIA treatment and consider repeating the test if initial CgA levels are high [see Drug Interactions (7)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in labeling:
- Hypersensitivity Reactions [see Warnings and Precautions (5.1)]
- Clostridioides difficile-Associated Diarrhea [see Warnings and Precautions (5.2)]
- Acute Interstitial Nephritis [see Warnings and Precautions (5.4)]
- Cutaneous and Systemic Lupus Erythematosus [see Warnings and Precautions (5.6)]
- Rash in Patients with Mononucleosis [see Warnings and Precautions (5.7)]
- Uveitis [see Warnings and Precautions (5.8)]
6.1 Clinical Trials Experience with TALICIA
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of TALICIA was assessed in adult patients who were screened and found to be positive for H. pylori infection in one active-controlled (Study 1) and one placebo-controlled (Study 2) clinical trial. Patients received TALICIA, amoxicillin and omeprazole, or placebo every eight hours for 14 consecutive days taken with food. A total of 305 patients received TALICIA in Studies 1 and 2, 227 patients received amoxicillin and omeprazole (as omeprazole magnesium) in Study 1, and 41 patients received placebo in Study 2. These patients had a mean age of 46.4 years (range 18 to 70 years); 62.3% were female, 80.3% were white with 64.2% Hispanic or Latino.
Adverse Reactions Leading to Discontinuation
Treatment discontinuation due to an adverse reaction occurred in 1% (4/305) of patients receiving TALICIA, <1% (1/227) of patients receiving amoxicillin and omeprazole, and 2% (1/41) of patients receiving placebo. Adverse reactions leading to discontinuation of TALICIA were nausea and vomiting, nausea, nasal congestion, and nasopharyngitis, in one patient each.
Most Common Adverse Reactions
Selected adverse reactions occurring in ≥1% of patients receiving TALICIA in Study 1 and 2 are described in Table 1.
Table 1: Selected Adverse Reactions Occurring in 1% or Greater of Patients Receiving TALICIA in Studies 1 and 2
a Headache includes: headache and migraine.
b Abdominal pain includes: abdominal pain, abdominal pain upper, and abdominal pain lower.
c Riboflavin was administered in Study 1 to prevent unintentional unblinding and may have contributed to under-reporting of chromaturia.
d Rash includes: rash, rash maculo-papular, rash morbilliform, and urticaria.
e Dyspepsia includes: dyspepsia and epigastric discomfort.
f Vulvovaginal candidiasis includes: vulvovaginal candidiasis, vulvovaginal mycotic infection, fungal infection, and vaginal discharge + vulvovaginal burning sensation + vulvovaginal pruritus.Study 1
Study 2
Adverse ReactionTALICIA
(N=228)
n (%)Amoxicillin and Omeprazole
(N=227)
n (%)TALICIA
(N=77)
n (%)Placebo
(N=41)
n (%)Diarrhea
23 (10.1)
18 (7.9)
11 (14.3)
4 (9.8)
Headachea
17 (7.5)
16 (7.0)
12 (15.6)
4 (9.8)
Nausea
11 (4.8)
12 (5.3)
3 (3.9)
1 (2.4)
Abdominal Painb
8 (3.5)
11 (4.8)
3 (3.9)
2 (4.9)
Chromaturiac
0
0
10 (13.0)
1 (2.4)
Rashd
6 (2.6)
2 (0.9)
4 (5.2)
0
Dyspepsiae
5 (2.2)
3 (1.3)
1 (1.3)
0
Vomiting
5 (2.2)
5 (2.2)
1 (1.3)
2 (4.9)
Oropharyngeal pain
2 (0.9)
2 (0.9)
3 (3.9)
0
Vulvovaginal candidiasisf
5 (2.2)
5 (2.2)
0
0
6.2 Other Important Adverse Reactions from the Labeling of the Individual Components of TALICIA
Additional adverse reactions that occurred in 1% or greater of patients treated with omeprazole or rifabutin alone in clinical trials were as follows:
Omeprazole
Flatulence, acid regurgitation, upper respiratory infection, constipation, dizziness, asthenia, back pain, and cough.
Rifabutin
Flatulence, asthenia, chest pain, fever, pain, leucopenia, anemia, anorexia, eructation, myalgia, insomnia, and taste perversion.
The following selected adverse reactions occurred in less than 1% of patients treated with rifabutin alone: flu-like syndrome, hepatitis, hemolysis, arthralgia, myositis, dyspnea, skin discoloration, thrombocytopenia, pancytopenia, and jaundice.
6.3 Post-Marketing Experience with Components of TALICIA
Because these reactions are voluntarily reported from a population of uncertain size, it is not always possible to reliably estimate their actual frequency or establish a causal relationship to drug exposure.
Omeprazole
Cardiovascular: angina, tachycardia, bradycardia, palpitations, elevated blood pressure, peripheral edema
Endocrine: gynecomastia
Gastrointestinal: pancreatitis including fatal pancreatitis, anorexia, irritable colon, fecal discoloration, mucosal atrophy of the tongue, stomatitis, abdominal swelling, dry mouth, microscopic colitis, fundic gland polyps, gastroduodenal carcinoids in patients with Zollinger-Ellison syndrome on long-term treatment as a manifestation of the underlying condition associated with such tumors
Hepatic: fatal hepatic failure or necrosis, hepatic encephalopathy, hepatocellular disease, cholestatic disease, mixed hepatitis, jaundice
Metabolism and Nutritional disorders: hypoglycemia, hypomagnesemia, with or without hypocalcemia and/or hypokalemia, hyponatremia, weight gain
Musculoskeletal: muscle weakness, myalgia, muscle cramps, joint pain, leg pain, bone fracture.
Nervous System/Psychiatric: depression, agitation, aggression, hallucinations, confusion, insomnia, nervousness, apathy, somnolence, anxiety, dream abnormalities, tremors, paresthesia, vertigo
Respiratory: epistaxis
Skin: photosensitivity, urticaria, pruritus, petechiae, purpura, alopecia, dry skin, hyperhidrosis
Special Senses: tinnitus, taste perversion
Ocular: optic atrophy, optic neuritis, dry eye syndrome, ocular irritation, blurred vision, double vision
Urogenital: hematuria, proteinuria, elevated serum creatinine, microscopic pyuria, urinary tract infection, glycosuria, urinary frequency, testicular pain
Hematologic: Agranulocytosis, hemolytic anemia, pancytopenia, neutropenia, anemia, thrombocytopenia, leukopenia, leukocytosis
Amoxicillin
Gastrointestinal: black hairy tongue
Liver: hepatic dysfunction, cholestatic jaundice, cholestasis, acute cytolytic hepatitis
Renal: crystalluria [see Overdosage (10)]
Hemic and Lymphatic Systems: anemia, hemolytic anemia, thrombocytopenia, thrombocytopenic purpura, eosinophilia, leukopenia, and agranulocytosis
Central Nervous System: hyperactivity, agitation, anxiety, insomnia, confusion, convulsions, behavioral changes, and/or dizziness
Rifabutin
Blood and lymphatic system disorders: agranulocytosis, lymphopenia
-
7 DRUG INTERACTIONS
7.1 Interactions with Other Drugs and Diagnostics
Drug interaction studies with TALICIA have not been conducted. The drug interaction information described here is based on the prescribing information of individual TALICIA components: omeprazole, amoxicillin, and rifabutin.
Rifabutin is a substrate and inducer of cytochrome P450 (CYP) 3A enzymes. Omeprazole is a substrate and an inhibitor of CYP2C19, and a substrate of CYP3A4. Co-administration of TALICIA and other drugs that are substrates, inhibitors, or inducers of these enzymes may alter concentrations of rifabutin/omeprazole or other co-administered drugs [See Table 2 below and Clinical Pharmacology (12.3)].
Omeprazole magnesium is a PPI. Refer to the prescribing information of the drugs used concomitantly with TALICIA for further information on their interactions with PPIs.
Table 2: Interactions with TALICIA When Co-Administered with Other Drugs and Diagnostics
CYP2C19 or CYP3A4 Inducers
Clinical Impact
Decreased exposure of omeprazole when used concomitantly with strong inducers.
Prevention or Management
St. John’s Wort, rifampin: Avoid concomitant use with TALICIA [see Warnings and Precautions (5.5)].
Ritonavir-containing products: See prescribing information for specific drugs.
CYP2C19 or CYP3A4 Inhibitors
Clinical Impact
Increased blood levels of omeprazole and rifabutin.
Prevention or Management
Voriconazole: Concomitant use with TALICIA is contraindicated [see Contraindications (4)].
Fluconazole, posaconazole and itraconazole: Avoid concomitant use with TALICIA. If coadministration cannot be avoided, monitor patients for rifabutin associated adverse events, and lack of anti-fungal efficacy.
CYP2C19 Substrates (e.g., Clopidogrel, citalopram, cilostazol, phenytoin, diazepam)
Clinical Impact
Increased plasma concentrations of CYP2C19 substrate drugs or decreased/increased plasma concentrations of its active metabolite(s) [see Clinical Pharmacology (12.3)].
Prevention or Management
Clopidogrel: Consider use of alternative anti-platelet therapy [see Warnings and Precautions (5.5)]. Avoid concomitant use with TALICIA.
Antiretrovirals/Protease Inhibitors
Clinical Impact
Antiretrovirals/protease inhibitors may increase rifabutin blood levels.
The effect of PPIs (such as omeprazole in TALICIA) on antiretroviral drugs is variable. The clinical importance and the mechanisms behind these interactions are not always known.
- Decreased exposure of some antiretroviral drugs (e.g., rilpivirine, atazanavir and nelfinavir) when used concomitantly with omeprazole may reduce antiviral effect and promote the development of drug resistance [see Clinical Pharmacology (12.3)].
- Increased exposure of other antiretroviral drugs (e.g., saquinavir) when used concomitantly with omeprazole may increase toxicity [see Clinical Pharmacology (12.3)].
There are other antiretroviral drugs which do not result in clinically relevant interactions with omeprazole.
Prevention or Management
Delavirdine: Combination treatment with TALICIA and delavirdine is contraindicated [see Contraindications (4)].
Rilpivirine-containing products: Concomitant use with TALICIA is contraindicated [see Contraindications (4)].
Avoid concomitant use of TALICIA with amprenavir, indinavir, lopinavir/ritonavir, saquinavir/ritonavir, ritonavir, tipranavir/ritonavir, fosamprenavir/ritonavir, or nelfinavir [see Warnings and Precautions (5.5)].
Other antiretrovirals: See prescribing information for specific antiretroviral drugs.
Probenecid
Clinical Impact
Increased and prolonged blood levels of amoxicillin.
Allopurinol
Clinical Impact
Increase in the incidence of rashes is reported in patients receiving both allopurinol and amoxicillin together compared to patients receiving amoxicillin alone. It is not known whether this potentiation of amoxicillin rashes is due to allopurinol or the hyperuricemia present in these patients.
Prevention or Management
Discontinue allopurinol at the first appearance of skin rash. Assess benefit-risk of continuing TALICIA treatment.
Warfarin, and Other Oral Anticoagulants
Clinical Impact
Abnormal prolongation of prothrombin time (increased international normalized ratio [INR]) has been reported in patients receiving amoxicillin and oral anticoagulants and in patients receiving PPIs, including omeprazole, and warfarin concomitantly.
Increases in INR and prothrombin time may lead to abnormal bleeding and even death.
Prevention or Management
Monitor INR and prothrombin time and adjust the dose of warfarin or other oral anticoagulants to maintain the desired level of anticoagulation.
Methotrexate
Clinical Impact
Concomitant use of omeprazole with methotrexate (primarily at high doses) may elevate and prolong serum levels of methotrexate and/or its metabolite hydroxymethotrexate, possibly leading to methotrexate toxicities [see Warnings and Precautions (5.5)].
Prevention or Management
Avoid concomitant use of TALICIA in patients receiving high-dose methotrexate.
Digoxin
Clinical Impact
Potential for increased digoxin blood levels [see Clinical Pharmacology (12.3)].
Prevention or Management
Monitor digoxin concentrations. Dose adjustment may be needed to maintain therapeutic drug concentrations. See digoxin prescribing information.
Drugs Dependent on Gastric pH for Absorption (e.g., iron salts, erlotinib, dasatinib, nilotinib, mycophenolate mofetil, ketoconazole/itraconazole)
Clinical Impact
Omeprazole can alter the absorption of other drugs due to its effect of reducing intragastric acidity thereby increasing gastric pH.
Prevention or Management
Mycophenolate mofetil (MMF): Use TALICIA with caution in transplant patients receiving MMF [see Clinical Pharmacology (12.3)].
See the prescribing information of other drugs dependent on gastric pH for absorption.
Tacrolimus
Clinical Impact
Potential for increased tacrolimus blood levels, especially in patients who are intermediate or poor metabolizers of CYP2C19.
Prevention or Management
Monitor tacrolimus whole blood levels and adjust dose as per the prescribing information for tacrolimus.
Drugs Metabolized via the CYP450 Enzymes (e.g., cyclosporine, disulfiram)
Clinical Impact
Interactions are reported with omeprazole and other drugs metabolized via the CYP450 enzymes.
Prevention or Management
Monitor patients to determine if it is necessary to adjust the dosage of these other drugs when taken concomitantly with TALICIA.
Oral Contraceptives
Clinical Impact
Concomitant use of amoxicillin and rifabutin with hormonal contraceptives may lead to loss of its efficacy due to lower estrogen reabsorption and decreased ethinylestradiol and norethindrone concentrations, respectively [see Warnings and Precautions (5.3)].
Prevention or Management
Patients should be advised to use additional or alternative non-hormonal methods of contraception.
Diagnostic Investigations for Neuroendocrine Tumors
Clinical Impact
PPI-induced decrease in gastric acidity may lead to increased serum chromogranin A (CgA) levels, which may cause false positive results in diagnostics for neuroendocrine tumors [see Warnings and Precautions (5.9)].
Prevention or Management
Assess CgA levels at least 14 days after stopping TALICIA treatment and consider repeating the test if initial CgA levels are high. If serial tests are performed (e.g., for monitoring), the same commercial laboratory should be used for testing, as reference ranges between tests may vary.
Urine Glucose Test
Clinical Impact
High urine concentrations of ampicillin or amoxicillin may result in false-positive reactions when using glucose tests based on the Benedict’s copper reduction reaction that determines the amount of reducing substances like glucose in the urine.
Prevention or Management
Glucose tests based on enzymatic glucose oxidase reactions should be used.
Interaction with Secretin Stimulation Test
Clinical Impact
Hyper-response in gastrin secretion in response to secretin stimulation test may falsely suggest gastrinoma.
Prevention or Management
Test should be performed at least 14 days after stopping TALICIA treatment to allow gastrin levels to return to baseline.
False Positive Urine Tests for Tetrahydrocannabinol (THC)
Clinical Impact
There have been reports of false positive urine screening tests for THC in patients receiving PPIs.
Prevention or Management
An alternative confirmatory method should be considered to verify positive results.
Other Laboratory Tests
Clinical Impact
Following administration of ampicillin or amoxicillin to pregnant women, a transient decrease in plasma concentration of total conjugated estriol, estriol-glucuronide, conjugated estrone, and estradiol has been noted.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on animal reproduction studies, TALICIA may cause fetal harm when administered to pregnant women. There are no adequate and well controlled studies of amoxicillin, omeprazole, or rifabutin (used separately or together) in pregnant women. Use of TALICIA is generally not recommended for use in pregnancy. If TALICIA is used during pregnancy, advise pregnant women of the potential risk to a fetus.
Omeprazole: Available epidemiologic data do not demonstrate an increased risk of major congenital malformations or other adverse pregnancy outcomes with first trimester omeprazole use. Reproduction studies in rats and rabbits resulted in dose-dependent embryo-lethality at omeprazole doses that were approximately 1.13 to 11 times an oral human dose of 120 mg.
Fetal malformations were not observed in animal reproduction studies with administration of oral esomeprazole (an enantiomer of omeprazole) magnesium in rats and rabbits during organogenesis with doses about 23 times and 14 times, respectively, of an oral human dose of 120 mg esomeprazole or omeprazole. Changes in bone morphology were observed in offspring of rats dosed through most of pregnancy and lactation at doses equal to or greater than approximately 11 times an oral human dose of 120 mg esomeprazole or omeprazole. When maternal administration was confined to gestation only, there were no effects on bone physeal morphology in the offspring at any age [see Data].
Amoxicillin: Available data from published epidemiologic studies and pharmacovigilance case reports over several decades with amoxicillin use have not established drug-associated risks of major birth defects, miscarriage, or adverse maternal or fetal outcomes [see Data]. No adverse developmental effects were observed in animal reproduction studies with administration of amoxicillin to pregnant mice and at doses up to 3 to 6 times an oral human dose of 3 grams.
Rifabutin: Fetal malformations were not observed in rat or rabbit reproduction studies given rifabutin at dose levels up to 200 mg/kg (6 to 13 times the recommended human dose). In rats, given rifabutin at 200 mg/kg/day (about 6 times the recommended human daily dose), there was a decrease in fetal viability. Increased skeletal anomalies were observed in rats and rabbits at 40 and 80 mg/kg/day, respectively (corresponding to approximately an equivalent dose and 5 times the recommended human daily dose); maternal toxicity was noted at 80 mg/kg in rabbits [see Data].
The estimated background risks of major birth defects and miscarriage for the indicated population are unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Human Data
Omeprazole
Four published epidemiological studies compared the frequency of congenital abnormalities among infants born to women who used omeprazole during pregnancy with the frequency of abnormalities among infants of women exposed to H2-receptor antagonists or other controls.
A population-based retrospective cohort epidemiological study from the Swedish Medical Birth Registry, covering approximately 99% of pregnancies, from 1995 to 99, reported on 955 infants (824 exposed during the first trimester with 39 of these exposed beyond first trimester, and 131 exposed after the first trimester) whose mothers used omeprazole during pregnancy. The number of infants exposed in utero to omeprazole that had any malformation, low birth weight, low Apgar score, or hospitalization was similar to the number observed in this population. The number of infants born with ventricular septal defects and the number of stillborn infants was slightly higher in the omeprazole-exposed infants than the expected number in this population.
A population-based retrospective cohort study covering all live births in Denmark from 1996 to 2009, reported on 1,800 live births whose mothers used omeprazole during the first trimester of pregnancy and 837,317 live births whose mothers did not use any proton pump inhibitor. The overall rate of birth defects in infants born to mothers with first trimester exposure to omeprazole was 2.9% and 2.6% in infants born to mothers not exposed to any proton pump inhibitor during the first trimester.
A retrospective cohort study reported on 689 pregnant women exposed to either H2-blockers or omeprazole in the first trimester (134 exposed to omeprazole) and 1,572 pregnant women unexposed to either during the first trimester. The overall malformation rate in offspring born to mothers with first trimester exposure to omeprazole, an H2-blocker, or were unexposed was 3.6%, 5.5%, and 4.1% respectively.
A small prospective observational cohort study followed 113 women exposed to omeprazole during pregnancy (89% with first trimester exposures). The reported rate of major congenital malformations was 4% in the omeprazole group, 2% in controls exposed to non-teratogens, and 2.8% in disease-paired controls. Rates of spontaneous and elective abortions, preterm deliveries, gestational age at delivery, and mean birth weight were similar among the groups.
Several studies have reported no apparent adverse short-term effects on the infant when single dose oral or intravenous omeprazole was administered to over 200 pregnant women as premedication for cesarean section under general anesthesia.
Amoxicillin
While available studies cannot definitively establish the absence of risk, published epidemiological data and postmarketing case reports have not reported a consistent association with amoxicillin and major birth defects, miscarriage, or adverse maternal or fetal outcomes when amoxicillin was used during pregnancy. Available studies have methodologic limitations, including small sample size, retrospective data collection, under-capture of non-live births, exposure misclassification and inconsistent comparator groups.
Rifabutin
Small retrospective observational studies evaluated the use of rifabutin (in combination with other drugs) for treatment of tuberculosis during pregnancy. Available studies were inconclusive in determining whether rifabutin use during pregnancy was associated with adverse effects in the pregnant woman or neonates.
Animal Data
Omeprazole
Reproductive studies conducted with omeprazole in rats at oral doses up to 138 mg/kg/day (about 11 times an oral human dose of 120 mg on a body surface area basis) and in rabbits at doses up to 69.1 mg/kg/day (about 11 times an oral human dose of 120 mg on a body surface area basis) during organogenesis did not show fetal malformations. In rabbits, omeprazole in a dose range of 6.9 to 69.1 mg/kg/day (about 1 to 11 times an oral human dose of 120 mg on a body surface area basis) administered during organogenesis produced dose-related increases in embryo-lethality, fetal resorptions, and pregnancy disruptions. In rats, dose-related embryo/fetal toxicity and postnatal developmental toxicity were observed in offspring resulting from parents treated with omeprazole at 13.8 to 138.0 mg/kg/day (about 1 to 11 times an oral human dose of 120 mg on a body surface area basis), administered prior to mating through the lactation period.
Esomeprazole
The data described below was generated from studies using esomeprazole, an enantiomer of omeprazole. The animal to human dose multiples are based on the assumption of equal systemic exposure to esomeprazole in humans following oral administration of either 120 mg esomeprazole or 120 mg omeprazole.
No effects on embryo-fetal development were observed in reproduction studies with esomeprazole magnesium in rats at oral doses up to 280 mg/kg/day (about 23 times an oral human dose of 120 mg on a body surface area basis) or in rabbits at oral doses up to 86 mg/kg/day (about 14 times an oral human dose of 120 mg esomeprazole or omeprazole on a body surface area basis) administered during organogenesis.
A pre-and postnatal developmental toxicity study in rats with additional endpoints to evaluate bone development was performed with esomeprazole magnesium at oral doses of 14 to 280 mg/kg/day (about 1 to 23 times an oral human dose of 120 mg esomeprazole or omeprazole on a body surface area basis). Neonatal/early postnatal (birth to weaning) survival was decreased at doses equal to or greater than 138 mg/kg/day (about 11 times an oral human dose of 120 mg esomeprazole or omeprazole on a body surface area basis). Body weight and body weight gain were reduced and neurobehavioral or general developmental delays in the immediate post-weaning timeframe were evident at doses equal to or greater than 69 mg/kg/day (about 6 times an oral human dose of 120 mg esomeprazole or omeprazole on a body surface area basis). In addition, decreased femur length, width and thickness of cortical bone, decreased thickness of the tibial growth plate and minimal to mild bone marrow hypocellularity were noted at doses equal to or greater than 14 mg/kg/day (about equivalent to the oral human dose of 120 mg esomeprazole or omeprazole on a body surface area basis). Physeal dysplasia in the femur was observed in offspring of rats treated with oral doses of esomeprazole magnesium at doses equal to or greater than 138 mg/kg/day (about 11 times an oral human dose of 120 mg esomeprazole or omeprazole on a body surface area basis).
Effects on maternal bone were observed in pregnant and lactating rats in the pre-and postnatal toxicity study when esomeprazole magnesium was administered at oral doses of 14 to 280 mg/kg/day (about 1 to 23 times an oral human dose of 120 mg esomeprazole or omeprazole on a body surface area basis). When rats were dosed from gestational day 7 through weaning on postnatal day 21, a statistically significant decrease in maternal femur weight of up to 14% (as compared to placebo treatment) was observed at doses equal to or greater than 138 mg/kg/day (about 11 times an oral human dose of 120 mg esomeprazole or omeprazole on a body surface area basis).
A pre-and postnatal development study in rats with esomeprazole strontium (using equimolar doses compared to esomeprazole magnesium study) produced similar results in dams and pups as described above.
A follow up developmental toxicity study in rats with further time points to evaluate pup bone development from postnatal day 2 to adulthood was performed with esomeprazole magnesium at oral doses of 280 mg/kg/day (about 23 times an oral human dose of 120 mg on a body surface area basis) where esomeprazole administration was from either gestational day 7 or gestational day 16 until parturition. When maternal administration was confined to gestation only, there were no effects on bone physeal morphology in the offspring at any age.
Amoxicillin
Reproduction studies have been performed in mice and rats at doses up to 2000 mg/kg (3 and 6 times the 3 g human dose, based on body surface area). There was no evidence of harm to the fetus due to amoxicillin.
Rifabutin
Reproduction studies have been carried out in rats and rabbits given rifabutin using dose levels up to 200 mg/kg (about 6 to 13 times the recommended human daily dose based on body surface area comparisons). No fetal malformations were observed in either species. In rats, given 200 mg/kg/day, (about 6 times the recommended human daily dose based on body surface area comparisons), there was a decrease in fetal viability. In rats, at 40 mg/kg/day (approximately equivalent to the recommended human daily dose based on body surface area comparisons), rifabutin caused an increase in fetal skeletal variations. In rabbits, at 80 mg/kg/day (about 5 times the recommended human daily dose based on body surface area comparisons), rifabutin caused maternal toxicity and increase in fetal skeletal anomalies.
8.2 Lactation
Risk Summary
Data from a published clinical lactation study reports that amoxicillin is present in human milk. Published adverse effects with amoxicillin exposure in the breast-fed infant include diarrhea. There are no data on the effects of amoxicillin on milk production. Limited data suggest omeprazole may be present in human milk. There are no clinical data on the effects of omeprazole on the breast-fed infant or on milk production. There are no data on the presence of rifabutin in human milk or the effects of rifabutin on the breast-fed infant or on milk production.
The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for TALICIA and any potential adverse effects on the breast-fed child from TALICIA or from the underlying condition.
8.3 Females and Males of Reproductive Potential
Contraception
Both rifabutin and amoxicillin components of TALICIA interact with hormonal contraceptives resulting in lower levels of these contraceptives. Therefore, female patients taking hormonal contraceptives should use an additional non-hormonal highly effective method of contraception while taking TALICIA [see Drug Interactions (7.1)].
Infertility
Males
Based on findings in rodents, TALICIA may impair fertility in males of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Safety and effectiveness of TALICIA in pediatric patients below the age of 18 years with H. pylori infection have not been established.
Esomeprazole, an enantiomer of omeprazole, was shown to decrease body weight, body weight gain, femur weight, femur length, and overall growth in juvenile rats at oral doses about 11 to 23 times a daily human dose of 120 mg esomeprazole or omeprazole based on body surface area [see Nonclinical Toxicology (13.2)].
8.5 Geriatric Use
Clinical studies of TALICIA did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger adult patients.
Omeprazole
Omeprazole was administered to over 2000 elderly individuals (≥ 65 years of age) in clinical trials in the U.S. and Europe. There were no differences in safety and effectiveness between the elderly and younger subjects. Other reported clinical experience has not identified differences in response between the elderly and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Amoxicillin
An analysis of clinical studies of amoxicillin was conducted to determine whether subjects aged 65 and older respond differently from younger subjects. These analyses have not identified differences in responses between the elderly and younger patients, but a greater sensitivity of some older individuals cannot be ruled out.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, it may be useful to monitor renal function in elderly patients taking TALICIA.
Rifabutin
Clinical studies of rifabutin did not include sufficient numbers of subjects aged 65 and older to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
8.6 Renal Impairment
It is recommended to avoid the use of TALICIA in patients with severe renal impairment (GFR < 30 mL/min). Amoxicillin is primarily eliminated by the kidney [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
It is recommended to avoid the use of TALICIA in patients with hepatic impairment. In patients with hepatic impairment (Child-Pugh Class A, B, or C) exposure to omeprazole substantially increased compared to healthy subjects [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
TALICIA
No information is available on accidental overdosage of TALICIA in humans.
In case of an overdose, patients should contact a physician, poison control center, or emergency room. The available overdosage information for each of the individual components in TALICIA (omeprazole, amoxicillin and rifabutin) are summarized below:
Omeprazole
There have been reports of overdosage with omeprazole in humans. Doses ranged up to 2400 mg (120 times the usual recommended clinical dose). Manifestations were variable, but included confusion, drowsiness, blurred vision, tachycardia, nausea, vomiting, diaphoresis, flushing, headache, dry mouth, and other adverse reactions similar to those seen in normal clinical experience [see Adverse Reactions (6.3)]. Symptoms were transient, and no serious clinical outcome has been reported when omeprazole was taken alone. No specific antidote for omeprazole overdosage is known. Omeprazole is extensively protein bound and is, therefore, not readily dialyzable. In the event of overdosage, treatment should be symptomatic and supportive.
Amoxicillin
In case of overdosage, discontinue medication, treat symptomatically, and institute supportive measures as required. A prospective study of 51 pediatric patients at a poison-control center suggested that overdosages of less than 250 mg/kg of amoxicillin are not associated with significant clinical symptoms.
Crystalluria, in some cases leading to renal failure, has also been reported after amoxicillin overdosage. In case of overdosage, adequate intake and diuresis should be maintained to reduce the risk of amoxicillin crystalluria.
Renal impairment appears to be reversible with cessation of drug administration. High blood concentrations may occur more readily in patients with impaired renal function because of decreased renal clearance of amoxicillin. Amoxicillin can be removed from circulation by hemodialysis.
Rifabutin
No information is available on accidental overdosage of rifabutin in humans.
While there is no experience in the treatment of overdose with rifabutin capsules, clinical experience with rifamycins suggests that gastric lavage to evacuate gastric contents (within a few hours of overdose), followed by instillation of an activated charcoal slurry into the stomach, may help adsorb any remaining drug from the gastrointestinal tract.
Rifabutin is 85% protein bound and distributed extensively into tissues (volume of distribution at steady state: 8 to 9 L/kg). It is not primarily excreted via the urinary route (less than 10% as unchanged drug); therefore, neither hemodialysis nor forced diuresis is expected to enhance the systemic elimination of unchanged rifabutin from the body in a patient with an overdose of rifabutin.
-
11 DESCRIPTION
TALICIA delayed-release capsules contain omeprazole magnesium, amoxicillin and rifabutin for oral administration. Omeprazole magnesium is included in the delayed-release component of the capsule, and amoxicillin and rifabutin are included in the immediate-release component of the capsule. Each delayed-release capsule contains:
- omeprazole 10 mg (equivalent to 10.3 mg of omeprazole magnesium)
- amoxicillin 250 mg (equivalent to 286.9 mg of amoxicillin trihydrate)
- rifabutin 12.5 mg
Omeprazole magnesium is a proton pump inhibitor. Amoxicillin and rifabutin are antibacterial drugs.
Each TALICIA delayed-release capsule contains the following inactive ingredients: crospovidone, FD&C Red 3, FD&C Yellow 6, gelatin, hydroxypropyl cellulose, hypromellose, magnesium stearate, mannitol-starch, methacrylic acid copolymer, meglumine, pregelatinized starch, silica, sodium bicarbonate, sodium lauryl sulfate, talc, titanium dioxide and triethyl citrate.
Omeprazole Magnesium
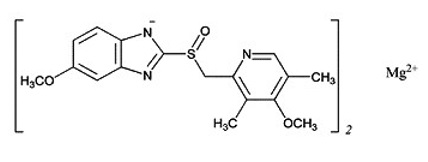
Omeprazole magnesium is a white to off-white powder with a melting point with degradation at 200 °C. The salt is slightly soluble (0.25 mg/mL) in water at 25 °C, and it is soluble in methanol. Omeprazole magnesium is 5-methoxy-2-[[(4-methoxy-3,5-dimethyl-2-pyridyl) methyl] sulfinyl]benzimidazole, (RS) magnesium salt (2:1). Omeprazole magnesium has a molecular formula of (C17H19N3O3S)2 Mg, and a molecular weight of 713.12. The structural formula is:
Amoxicillin
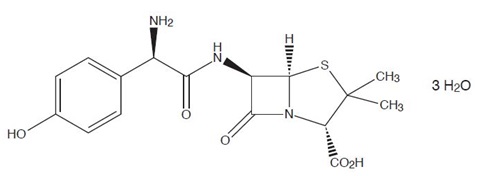
Amoxicillin is a semisynthetic antibacterial drug, an analog of ampicillin. Chemically it is (2S,5R,6R)-6-[(R)-(-)-2- amino-2-(p-hydroxyphenyl)acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0] heptane-2-carboxylic acid trihydrate. Amoxicillin has a molecular formula of C16H19N3O5S3 H2O, and a molecular weight of 419.45. The structural formula is:
Rifabutin
Rifabutin is a red-violet powder soluble in chloroform and methanol, sparingly soluble in ethanol, and very slightly soluble in water (0.19 mg/mL). Its log P value (the base 10 logarithm of the partition coefficient between n-octanol and water) is 3.2 (n-octanol/water).
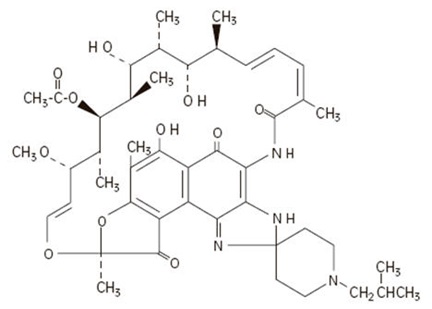
Rifabutin is (9S,12E,14S,15R,16S,17R,18R,19R,20S,21S,22E,24Z)-6-16,18,20-tetrahydroxy-1'-isobutyl-14-methoxy-7,9,15,17,19,21,25-heptamethylspiro [9,4-(epoxypentadeca[1,11,13]trienimino)-2H-furo[2',3':7,8]naphth[1,2-d] imidazole-2,4'-piperidine]-5,10,26-(3H,9H)-trione-16-acetate. Rifabutin has a molecular formula of C46H62N4O11, and a molecular weight of 847.02. The structural formula is:
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
TALICIA is a combination of antibacterial drugs (rifabutin, amoxicillin) and a proton pump inhibitor (omeprazole as omeprazole magnesium), an antisecretory drug [see Microbiology (12.4)].
12.2 Pharmacodynamics
After oral administration, the onset of the antisecretory effect of omeprazole occurs within one hour, with the maximum effect occurring within two hours. The antisecretory effect lasts longer than would be expected from the short (approximately one hour) plasma half-life, apparently due to prolonged binding to the parietal H+/K+ ATPase enzyme. When the drug is discontinued, secretory activity returns gradually, over 3 to 5 days. The inhibitory effect of omeprazole on acid secretion increases with repeated daily dosing.
12.3 Pharmacokinetics
The pharmacokinetic parameters of the components of TALICIA are summarized in Table 3.
Table 3: Mean (Standard Deviation) Pharmacokinetic Parameters of the Components of TALICIA
Cmax =Maximum plasma concentration, AUC24 =Area under the concentration vs. 24-hour time curve
a Cmax and AUC24, estimates derived from 15 healthy subjects following administration of four TALICIA capsules three times in a day (8 hours apart) resulting in the total daily oral doses of 150 mg rifabutin, 3000 mg amoxicillin, and 120 mg omeprazole.Pharmacokinetic Parametersa
Amoxicillin
Omeprazole
Rifabutin
Cmax (ng/mL)
15,860 (3,340)
1,281 (518)
88 (21)
AUC24 (ng*hr/mL)
145,788 (29,846)
7,161 (3,533)
1,320 (307)
The absorption, distribution, and elimination related pharmacokinetic information on the components of TALICIA are provided in Table 4.
Table 4: Pharmacokinetic Properties of the Components of TALICIA
Tmax = Time to reach Cmax, AUC∞ = Area under the concentration vs. time profile extrapolated to infinity, t1/2 = Elimination half-life, ↑ indicates increase, ↓ indicates decrease, ↔ indicates no significant change.
a Changes in Cmax, AUC∞, and Tmax estimates reported from a crossover food-effect study in 18 healthy subjects following the administration of four TALICIA capsules administered once with a high-fat, high calorie meal consisting approximately 1000 kcal (14% from protein, 53% from fat, and 33% from carbohydrates) compared to when four TALICIA capsules administered without food. Reported Tmax and t1/2 estimates are from the same study from 18 subjects (for rifabutin, from 17 subjects) who received TALICIA capsules without food.Pharmacokinetic Parameters
Amoxicillin
Omeprazole
Rifabutin
Absorption
Tmax (h), median (range)a
2 (1.25-3)
1.25 (0.75-1.77)
3 (2-6)
Effect of Food:
With high-fat meala (relative to fasting)
↓30% in Cmax
↔ AUC∞
↑Tmax by 1.5 hr
↓92% in Cmax
↓83% in AUC∞
↑Tmax by 3 hr
↑14% in Cmax
↑23% in AUC∞
↑Tmax by 2 hr
Distribution
Protein Binding
20%
95%
85%
Elimination
t1/2 (h), mean (standard deviation)
1.4 (0.2)
1 (0.3)
34 (25)
Metabolism
Metabolic pathways
Not significantly metabolized
- Extensively metabolized
- CYP2C19 (major) responsible for the formation of hydroxyomeprazole
- CYP3A4 (minor) responsible for the formation of omeprazole-sulphone
- These metabolites have very little or no antisecretory activity
- Of the five metabolites that have been identified, 25-O-desacetyl and 31-hydroxy are the most predominant with a plasma metabolite: parent AUC ratio of 0.10 and 0.07, respectively
- 25-O-desacetylrifabutin has an activity equal to the parent drug with up to 10% to the total antimicrobial activity
Excretion
Major route of elimination
60% of oral dose excreted in urine in 6-8 hours (mostly as unchanged drug)
77% of dose excreted in urine as metabolites and the remainder of the dose recovered in feces
- 53% of the oral dose was excreted in urine, primarily as metabolites
- About 30% of the dose is excreted in feces
- Renal and biliary clearance of unchanged drug each contribute approximately 5% to apparent oral clearance
Renal Impairment
For omeprazole, no clinically meaningful change in bioavailability was reported in patients with chronic renal impairment (CLcr between 10-62 mL/min/1.73 m2).
Amoxicillin is primarily eliminated by the kidney [see Use in Specific Populations (8.6)].
For rifabutin, the disposition was studied following 300 mg dose in 18 patients with varying degrees of renal function. Area under plasma concentration time curve (AUC) of rifabutin increased by about 71% in patients with severe renal impairment (CLcr <30 mL/min) compared to patients with creatinine clearance (CLcr) between 61-74 mL/min. In patients with mild to moderate renal impairment (CLcr between 30-61 mL/min), the AUC of rifabutin increased by about 41%.
Hepatic Impairment
The pharmacokinetics of amoxicillin and rifabutin in patients with moderate and severe hepatic impairment are not known.
For omeprazole, in patients with chronic hepatic disease classified as Child-Pugh Class A (n=3), B (n=4), and C (n=1), the bioavailability increased to approximately 100% compared to healthy subjects, reflecting decreased first-pass effect, and the plasma half-life of the drug increased to nearly 3 hours compared with the half-life in healthy subjects of 0.5 to 1 hour. Plasma clearance averaged 70 mL/min, compared with a value of 500 to 600 mL/min in healthy subjects [see Use in Specific Populations (8.7)].
Drug Interactions
Drug interaction studies with TALICIA have not been conducted. The drug interaction information described here is based on the prescribing information of individual TALICIA components: rifabutin, omeprazole magnesium, and amoxicillin [see Drug Interactions (7)].
Effect of Omeprazole on Other Drugs
Omeprazole is a time-dependent inhibitor of CYP2C19 and can increase the systemic exposure of co-administered drugs that are CYP2C19 substrates. In addition, administration of omeprazole increases intragastric pH and can alter the systemic exposure of certain drugs that exhibit pH-dependent solubility [see Drug Interactions (7)].
Effect of Rifabutin on Other Drugs
Multiple dosing of rifabutin has been associated with induction of hepatic metabolic enzymes of the CYP3A subfamily. Rifabutin’s predominant metabolite (25-desacetyl rifabutin) may also contribute to this effect. Metabolic induction due to rifabutin is likely to produce a decrease in plasma concentrations of concomitantly administered drugs that are primarily metabolized by the CYP3A enzymes. Similarly, concomitant medications that competitively inhibit the CYP3A activity may increase plasma concentrations of rifabutin [see Drug Interactions (7)].
Drug Interactions Between TALICIA Components
CYP enzymes are involved in the metabolism of omeprazole; therefore, rifabutin mediated induction of CYP enzymes is expected to reduce systemic exposure to omeprazole.
Table 5 and Table 6 summarizes the drug interactions information from the prescribing information of omeprazole and rifabutin, respectively.
Table 5: Summary of Drug Interaction Studies with Omeprazole
↑ indicates increase, ↓ indicates decrease, ND=No data, AUC=Area under the concentration vs. time curve, Cmax =Maximum serum/plasma concentrations, Cmin = Minimum serum/plasma concentrations.
‡ 3,4-dihydro-cilostazol has 4-7 times the activity of cilostazolCoadministered drug
Dosing regimen of coadministered drug
Dosing regimen of Omeprazole
Results
Rilpivirine
Multiple doses of 150 mg/day
Multiple doses of 20 mg/day
Rilpivirine: ↓40% AUC, ↓40% Cmax, and ↓33% Cmin
Nelfinavir
Multiple doses of 1250 mg twice daily
Multiple doses of 40 mg/day
Nelfinavir: ↓36% AUC, ↓37% Cmax, and ↓39% Cmin
M8: ↓92% in AUC, ↓89% Cmax, and ↓75% Cmin
Atazanavir
Multiple doses of 400 mg/day
Multiple doses of 40 mg/day
Atazanavir: ↓94% AUC, ↓96% Cmax, and ↓95% Cmin
Saquinavir
Saquinavir/ritonavir (1000/100 mg) twice daily for 15 days
40 mg/day on Days 11 to 15
Saquinavir: ↑82% AUC, ↑75% Cmax, and ↑106% Cmin
Clopidogrel
Three separate studies with 300 mg loading dose + 75 mg/day
80 mg/day at the same time as clopidogrel in two studies and 12 hours apart in the third studies
Summary results from three studies:
- ↓41% to 46% in the exposure to the active metabolite of clopidogrel from Day 1.
- Administration of clopidogrel and omeprazole at different times does not prevent interaction
Mycophenolate Mofetil (MMF)
1000 mg dose after the last dose of omeprazole
20 mg twice daily for four days
Mycophenolic acid (MPA)- active metabolite of MMF: ↓23% AUC and ↓52% Cmax
Cilostazol
ND
40 mg/day for a week
Cilostazol: ↑26% AUC and ↑18% Cmax
3,4-dihydro-cilostazol‡: ↑69% AUC and ↑29% Cmax
Diazepam
0.1 mg/kg given intravenously
20 mg/day concomitantly
Diazepam: ↓27% clearance and ↑36% half-life
Digoxin
ND
20 mg/day concomitantly
Digoxin: Up to ↑30% bioavailability
Voriconazole
400 mg twice daily for one day + 200 mg/day for 6 days
40 mg/day for a week
Voriconazole: Four-fold ↑ in AUC and two-fold ↑in Cmax
Table 6: Summary of Drug Interaction Studies with Rifabutin
↑ indicates increase, ↓ indicates decrease, ↔ indicates no significant change, ND = No data, AUC=Area under the concentration vs. time curve, Cmax = Maximum serum/plasma concentrations, Cmin = Minimum serum/plasma concentrations.
a Compared to rifabutin 300 mg once daily alone
b Compared to historical control (fosamprenavir/ritonavir 700/100 mg twice daily)
c Also taking zidovudine 500 mg once daily
d Compared to rifabutin 150 mg once daily alone
e Compared to rifabutin 300 mg once daily alone
f Data from a case report
g Compared to voriconazole 200 mg twice daily aloneCoadministered drug
Dosing regimen of coadministered drug
Dosing regimen of Rifabutin
Study population (n)
Effect on rifabutin
Effect on coadministered drug
ANTIVIRALS
Amprenavir
1200 mg twice daily x 10 days
300 mg once daily x 10 days
Healthy male subjects (6)
193%↑ AUC,
119%↑ Cmax
↔
Delavirdine
400 mg TID
300 mg once daily
HIV-infected patients (7)
230%↑ AUC,
128%↑ Cmax
80%↓ AUC,
75%↓ Cmax,
17%↓ Cmin
Didanosine
167 or 250 mg twice daily x 12 days
300 or 600 mg once daily x 1
HIV-infected patients (11)
↔
↔
Fosamprenavir/
ritonavir
700 mg twice daily plus ritonavir 100 mg twice daily x 2 weeks
150 mg every other day x 2 weeks
Healthy subjects (15)
↔ AUCa
15%↓ Cmax
35%↑ AUCb,
36%↑ Cmax,
36%↑ Cmin
Indinavir
800 mg TID x 10 days
300 mg once daily x 10 days
Healthy subjects (10)
173%↑ AUC,
134%↑ Cmax
34%↓ AUC,
25%↓ Cmax,
39%↓ Cmin
Lopinavir/
ritonavir
400/100 mg twice daily x 20 days
150 mg once daily x 10 days
Healthy subjects (14)
203%c ↑ AUC
112%↓Cmax
↔
Saquinavir/
ritonavir
1000/100 mg twice daily x 14 or 22 days
150 mg every 3 days x 21-22 days
Healthy subjects
53%↑ AUC d,
88% ↑ Cmax,
(n=11)
13%↓ AUC,
15%↓ Cmax,
(n=19)
Ritonavir
500 mg twice daily x 10 days
150 mg once daily x 16 days
Healthy subjects (5)
300%↑ AUC,
150%↑ Cmax
ND
Tipranavir/
ritonavir
500/200 mg twice daily x 15 doses
150 mg single dose
Healthy subjects (20)
190%↑ AUC,
70% ↑ Cmax
↔
Nelfinavir
1250 mg twice daily x 7-8 days
150 mg once daily x 8 days
HIV-infected patients (11)
83%↑ AUC e,
19%↑ Cmax
↔
Zidovudine
100 or 200 mg q4h
300 or 450 mg once daily
HIV-infected patients (16)
↔
32%↓ AUC,
48%↓ Cmax
ANTIFUNGALS
Fluconazole
200 mg once daily x 2 weeks
300 mg once daily x 2 weeks
HIV-infected patients (12)
82%↑ AUC,
88% ↑ Cmax
↔
Posaconazole
200 mg once daily x 10 days
300 mg once daily x 17 days
Healthy subjects (8)
72%↑ AUC,
31%↑ Cmax
49%↓ AUC,
43%↓ Cmax
Itraconazole
200 mg once daily
300 mg once daily
HIV-Infected patients (6)
↑f
70%↓ AUC,
75%↓ Cmax
Voriconazole
400 mg twice daily x 7 days (maintenance dose)
300 mg once daily x 7 days
Healthy male subjects (12)
331%↑ AUC,
195%↑ Cmax
~100%↑ AUC,
~100%↑ Cmax g
ANTI-PCP (Pneumocystis carinii pneumonia)
Dapsone
50 mg once daily
300 mg once daily
HIV-infected patients (16)
ND
27-40%↓ AUC
Sulfamethoxazole-Trimethoprim
800/160 mg
300 mg once daily
HIV-infected patients (12)
↔
15-20%↓ AUC
ANTI-MAC (Mycobacterium avium intracellulare complex)
Azithromycin
500 mg once daily x 1 day, then 250 mg once daily x 9 days
300 mg once daily
Healthy subjects (6)
↔
↔
Clarithromycin
500 mg twice daily
300 mg once daily
HIV-infected patients (12)
75%↑ AUC
50%↓ AUC
ANTI-TB (Tuberculosis)
Ethambutol
1200 mg
300 mg once daily x 7 days
Healthy subjects (10)
ND
↔
Isoniazid
300 mg
300 mg once daily x 7 days
Healthy subjects (6)
ND
↔
OTHER
Methadone
20-100 mg once daily
300 mg once daily x 13 days
HIV-infected patients (24)
ND
↔
Ethinylestradiol (EE)/
Norethindrone (NE)
35 mg EE / 1 mg NE x 21 days
300 mg once daily x 10 days
Healthy female subjects (22)
ND
EE:35%↓ AUC, 20%↓ Cmax,
NE: 46%↓ AUC
Theophylline
5 mg/kg
300 mg x 14 days
Healthy subjects (11)
ND
↔
12.4 Microbiology
Mechanism of Action
Amoxicillin acts through the inhibition of cell wall biosynthesis that leads to the death of bacteria.
Rifabutin inhibits DNA-dependent RNA polymerase in susceptible microorganisms but not in mammalian cells.
Mechanism of Resistance
Resistance to amoxicillin is mediated primarily through beta-lactamases that cleave the beta-lactam ring of amoxicillin, rendering it inactive.
Resistance to rifabutin occurs through mutations in the DNA-dependent RNA polymerase.
Antimicrobial Activity
TALICIA therapy has been shown to be active against most clinical isolates of H. pylori. In the clinical trials, 6.4% of the pretreatment isolates had amoxicillin MIC values >0.125 mcg/mL; 17.4% had clarithromycin MIC values ≥1 mcg/mL; 43.6% had metronidazole MIC values >8 mcg/mL. All isolates had rifabutin MIC values of <1 mcg/mL. The clinical significance of these MIC values is unknown.
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
Effects on Gastrointestinal Microbial Ecology
Decreased gastric acidity due to any means including proton pump inhibitors, increases gastric counts of bacteria normally present in the gastrointestinal tract. Treatment with proton pump inhibitors may lead to slightly increased risk of gastrointestinal infections due to pathogens such as Salmonella and Campylobacter and, in hospitalized patients, possibly also due to Clostridioides difficile.
12.5 Pharmacogenomics
CYP2C19, a polymorphic enzyme, is involved in the metabolism of omeprazole. The CYP2C19*1 allele is fully functional while the CYP2C19*2 and *3 alleles are nonfunctional. There are other alleles associated with no or decreased CYP2C19 function. Patients carrying two fully functional alleles are normal metabolizers and those carrying two nonfunctional alleles are poor metabolizers. The systemic exposure to omeprazole varies with a patient’s metabolism status: poor metabolizers > intermediate metabolizers > normal metabolizers. Approximately 3% of Caucasians and 15 to 20% of Asians are CYP2C19 poor metabolizers.
In a pharmacokinetic study of single 20 mg omeprazole dose, the AUC of omeprazole in Asian subjects was approximately four times higher than in Caucasians. In Study 1, the safety and tolerability of TALICIA was not substantially different in CYP2C19 poor metabolizers (n = 5) and intermediate metabolizers (n = 48) compared to normal metabolizers (n = 114).
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No long-term studies have been performed to evaluate the effect of TALICIA on carcinogenesis, mutagenesis, or impairment of fertility.
Omeprazole
In two 24-month carcinogenicity studies in rats, omeprazole at daily doses of 1.7, 3.4, 13.8, 44.0 and 140.8 mg/kg/day (about 0.1 to 11.4 times a human dose of 120 mg/day, as expressed on a body surface area basis) produced gastric enterochromaffin-like (ECL) cell carcinoids in a dose-related manner in both males and female rats; the incidence of this effect was markedly higher in female rats, which had higher blood concentrations of omeprazole. Gastric carcinoids seldom occur in the untreated rat. In addition, ECL cell hyperplasia was present in all treated groups of both sexes. In one of these studies, female rats were treated with 13.8 mg omeprazole/kg/day (about 1 times a human dose of 120 mg/day, based on body surface area) for one year, and then followed for an additional year without the drug. No carcinoids were seen in these rats. An increased incidence of treatment-related ECL cell hyperplasia was observed at the end of one year (94% treated vs. 10% controls). By the second year the difference between treated and control rats was much smaller (46% vs. 26%) but still showed more hyperplasia in the treated group. Gastric adenocarcinoma was seen in one rat (2%). No similar tumor was seen in male or female rats treated for two years. For this strain of rat, no similar tumor has been noted historically, but a finding involving only one tumor is difficult to interpret.
In a 52-week toxicity study in Sprague-Dawley rats, brain astrocytomas were found in a small number of males that received omeprazole at dose levels of 0.4, 2, and 16 mg/kg/day (about < 0.1 to 1.3 times the human dose of 120 mg/day, based on a body surface area basis). No astrocytomas were observed in female rats in this study. In a 2-year carcinogenicity study in Sprague-Dawley rats, no astrocytomas were found in males or females at the high dose of 140.8 mg/kg/day (about 11 times the human dose of 120 mg/day on a body surface area basis). A 78-week mouse carcinogenicity study of omeprazole did not show increased tumor occurrence, but the study was not conclusive. A 26-week p53 (+/–) transgenic mouse carcinogenicity study was not positive.
Omeprazole was positive for clastogenic effects in an in vitro human lymphocyte chromosomal aberration assay, in one of two in vivo mouse micronucleus tests, and in an in vivo bone marrow cell chromosomal aberration assay. Omeprazole was negative in the in vitro Ames test, an in vitro mouse lymphoma cell forward mutation assay, and an in vivo rat liver DNA damage assay.
Omeprazole at oral doses up to 138 mg/kg/day in rats (about 11 times the human dose of 120 mg on a body surface area basis) was found to have no effect on fertility and reproductive performance.
In 24-month carcinogenicity studies in rats, a dose-related significant increase in gastric carcinoid tumors and ECL cell hyperplasia was observed in both male and female animals. Carcinoid tumors have also been observed in rats subjected to fundectomy or long-term treatment with other proton pump inhibitors or high doses of H2-receptor antagonists.
Amoxicillin
Long-term studies in animals have not been performed to evaluate carcinogenic potential. Studies to detect mutagenic potential of amoxicillin alone have not been conducted; however, the following information is available from tests on a 4:1 mixture of amoxicillin and potassium clavulanate. Amoxicillin and potassium clavulanate were non-mutagenic in the Ames bacterial mutation assay, and the yeast gene conversion assay. Amoxicillin and potassium clavulanate were weakly positive in the mouse lymphoma assay, but the trend toward increased mutation frequencies in this assay occurred at doses that were also associated with decreased cell survival. Amoxicillin and potassium clavulanate were negative in the mouse micronucleus test and in the dominant lethal assay in mice. Potassium clavulanate alone was tested in the Ames bacterial mutation assay and in the mouse micronucleus test and was negative in each of these assays. In a multi-generation reproduction study in rats, no impairment of fertility or other adverse reproductive effects were seen at doses up to 500 mg/kg (approximately 2 times the 3 g human dose based on body surface area).
Rifabutin
Long-term carcinogenicity studies were conducted with rifabutin in mice and in rats. Rifabutin was not carcinogenic in mice at doses up to 180 mg/kg/day, or approximately 36 times the recommended human daily dose. Rifabutin was not carcinogenic in the rat at doses up to 60 mg/kg/day, about 12 times the recommended human dose.
Rifabutin was not mutagenic in the bacterial mutation assay (Ames Test) using both rifabutin-susceptible and resistant strains. Rifabutin was not mutagenic in Schizosaccharomyces pombe P1 and was not genotoxic in V-79 Chinese hamster cells, human lymphocytes in vitro, or mouse bone marrow cells in vivo.
Fertility was impaired in male rats given 160 mg/kg (32 times the recommended human daily dose).
13.2 Animal Toxicology and/or Pharmacology
A 28-day toxicity study with a 14-day recovery phase was conducted in juvenile rats with esomeprazole magnesium at doses of 70 to 280 mg/kg/day (about 6 to 23 times a daily oral human dose of 120 mg esomeprazole or omeprazole on a body surface area basis). An increase in the number of deaths at the high dose of 280 mg/kg/day was observed when juvenile rats were administered esomeprazole magnesium from postnatal day 7 through postnatal day 35. In addition, doses equal to or greater than 140 mg/kg/day (about 11 times a daily oral human dose of 120 mg esomeprazole or omeprazole on a body surface area basis), produced treatment-related decreases in body weight (approximately 14%) and body weight gain, decreases in femur weight and femur length, and affected overall growth. Comparable findings described above have also been observed in this study with another esomeprazole salt, esomeprazole strontium, at equimolar doses of esomeprazole.
-
14 CLINICAL STUDIES
The effectiveness and safety of TALICIA were evaluated in a randomized, double-blind, controlled study of TALICIA in treatment-naïve H. pylori-positive adult patients complaining of epigastric pain/discomfort (Study 1, NCT03198507). H. pylori infection at baseline was defined as positive by 13C urea breath test (UBT) and follow-up upper endoscopy (culture, histology, or Campylobacter-like organism test). Patients were randomized 1:1 to TALICIA or control (total daily dose of amoxicillin 3000 mg and omeprazole 120 mg) administered for 14 consecutive days. The trial was performed in the U.S and designed to evaluate the added contribution of rifabutin to the TALICIA triple combination.
H. pylori eradication was confirmed with a negative 13C UBT or fecal antigen test performed ≥28 days post-therapy. Patients with negative test results were considered treatment successes. Patients who tested positive for H. pylori infection were considered treatment failures, and patients with indeterminate, not assessable, or missing results from the test of cure visits underwent a repeat 13C UBT test. Persistent indeterminate results and patients without any 13C UBT or fecal antigen test after baseline were considered as treatment failures.
H. pylori eradication rates are shown in Table 7. The difference in response rates between TALICIA and the control was 26.1% (95% CI; 18.0, 34.1).
Table 7. Eradication Rates of H. pylori in Study 1
a The Intent to Treat (ITT) population included all randomized patients who received at least one dose of study drug.
b Of those subjects classified as treatment failures, all but one subject in the TALICIA group were positive by 13C UBT; this one subject was classified as a treatment failure due to a missing post-baseline test result.H. pylori Eradication
ITT Populationa
TALICIA
N = 228 (%)Control
N =227 (%)Success
191 (83.8)
131 (57.7)
Failure
37 (16.2)b
96 (42.3)
P-value
< 0.0001
A randomized, double-blind, placebo-controlled study of TALICIA in H. pylori-positive adult patients complaining of epigastric pain/discomfort (Study 2, NCT01980095) was performed in the U.S. and provided supportive evidence for the efficacy of TALICIA for the treatment of H. pylori infection; 77 patients taking TALICIA and 41 patients taking placebo were included in the ITT population, with an eradication rate of 76.6% (95% CI; 66.0%, 84.7%) for the TALICIA-treated patients compared to 2.4% for the placebo-treated patients. Eleven patients in the TALICIA arm and four patients in the placebo arm were classified as treatment failures due to missing 13C UBT results at the test-of-cure visit.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
TALICIA is supplied as an orange, opaque capsule containing omeprazole 10 mg (equivalent to omeprazole magnesium 10.3 mg), amoxicillin 250 mg and rifabutin 12.5 mg with “RHB” imprinted in black on the capsule cap and “105” imprinted in black on the capsule body. TALICIA capsules are supplied in a carton containing two bottles of 84 capsules each.
NDC: 57841-1150-1 Bottle containing 84 capsules
NDC: 57841-1150-2 Carton containing 2 Bottles of 84 capsules
Store at 20°C to 25°C (68°F to 77°F) [See USP Controlled Room Temperature].
Store and Dispense in original container with a child-resistant closure. Keep bottle tightly closed.
-
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise the patient to call their healthcare provided immediately if they develop new rash, skin lesions, muscle or joint pains, swelling, severe flu-like symptoms, difficulty breathing or visual symptoms.
Diarrhea
Counsel patients that diarrhea is a common problem caused by antibiotics, and it usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools even as late as 2 or more months after having taken their last dose of the antibiotic. Patients should contact their physician as soon as possible if they experience bloody diarrhea, persistent abdominal pain, fever, or chronic diarrhea that does not resolve.
Brown-Orange Discoloration
Urine, feces, saliva, sputum, perspiration, tears, and skin may be colored brown-orange due to the rifabutin component of TALICIA and some of its metabolites. Soft contact lenses may be permanently stained. Advise patients to be treated with TALICIA of these possibilities and counsel the patient that these should resolve after therapy is completed.
Drug Interactions
Advise patients not to take St. John’s Wort, amoxicillin or other penicillin products, rifabutin or other rifamycins, over-the counter (OTC) omeprazole or other PPIs during treatment with TALICIA. Advise patients that they should not start any new medication while taking TALICIA without first speaking with their health care provider.
Contraception
Counsel females of reproductive potential who are taking oral or other forms of hormonal birth-control medications to use an additional non-hormonal highly effective method of contraception while taking TALICIA.
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential that TALICIA is not recommended during pregnancy because of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy
Cutaneous or Systemic Lupus
Advise patients to report any symptoms associated with cutaneous or systemic lupus erythematosus.
Acute Interstitial Nephritis
Advise the patient or caregiver to call the patient’s healthcare provider immediately if they experience signs and/or symptoms associated with acute interstitial nephritis
Important Administration Instructions for TALICIA
- Advise patients to take four (4) TALICIA capsules every eight hours with food for 14 days. They should NOT crush or chew capsules.
- Advise patients to swallow TALICIA with at least 8 ounces of water.
- Advise patients not to take TALICIA with alcohol.
- Missed doses: Advise patients that if a dose is missed, administer as soon as possible. However, if the next scheduled dose is due, take the next dose on time. Do not take two doses at one time to make up for a missed dose. It is important for patients to complete the entire course of therapy.
- Counsel patients that antacids may be used concomitantly with TALICIA.
- Counsel patients to continue the full course of TALICIA regardless of whether or not their symptoms improve. Although change in dyspepsia symptoms may occur (either improvement or worsening), these are unlikely to be related to the H. pylori infection. Counsel patients that treatment of H. pylori infection is important due to its association with stomach ulcers, atrophic gastritis and increased risk of gastric cancer.
Antibacterial Resistance
Patients should be counseled that antibacterial drugs including TALICIA should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When TALICIA is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by TALICIA or other antibacterial drugs in the future.
TALICIA is distributed by RedHill Biopharma Inc. Raleigh, NC
TALICIA is manufactured in Sweden for RedHill Biopharma Ltd. Tel Aviv, Israel
TALICIA is a trademark registered with the U.S. Patent and Trademark Office and used under license by RedHill Biopharma Inc.
© 2019 RedHill Biopharma Ltd. All rights reserved.
-
PRINCIPAL DISPLAY PANEL - Carton
NDC: 57841-1150-2
Rx OnlyTalicia®
(omeprazole magnesium,
amoxicillin, and rifabutin)
delayed-release capsules
10 mg*/250 mg/12.5 mg2 BOTTLES x
84 CAPSULES
-
PRINCIPAL DISPLAY PANEL - Bottle Label
NDC: 57841-1150-1
Talicia®
(omeprazole magnesium,
amoxicillin, and rifabutin)
delayed-release capsules
10 mg*/250 mg/12.5 mgRx Only
84 CAPSULES
RedHill
Biopharma
-
INGREDIENTS AND APPEARANCE
TALICIA
omeprazole magnesium, amoxicillin and rifabutin capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 57841-1150 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OMEPRAZOLE MAGNESIUM (UNII: 426QFE7XLK) (OMEPRAZOLE - UNII:KG60484QX9) OMEPRAZOLE 10 mg AMOXICILLIN (UNII: 804826J2HU) (AMOXICILLIN ANHYDROUS - UNII:9EM05410Q9) AMOXICILLIN ANHYDROUS 250 mg RIFABUTIN (UNII: 1W306TDA6S) (RIFABUTIN - UNII:1W306TDA6S) RIFABUTIN 12.5 mg Inactive Ingredients Ingredient Name Strength CROSPOVIDONE (15 MPA.S AT 5%) (UNII: 68401960MK) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) HYPROMELLOSES (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MANNITOL (UNII: 3OWL53L36A) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) MEGLUMINE (UNII: 6HG8UB2MUY) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM BICARBONATE (UNII: 8MDF5V39QO) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) Product Characteristics Color orange Score no score Shape capsule Size 20mm Flavor Imprint Code RHB;105 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 57841-1150-2 2 in 1 CARTON 03/09/2020 1 NDC: 57841-1150-1 84 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA213004 03/09/2020 Labeler - RedHill Biopharma Ltd (533278342)
Trademark Results [Talicia]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 TALICIA 88675178 not registered Live/Pending |
RedHill Biopharma, Ltd. 2019-10-31 |
 TALICIA 88391531 not registered Live/Pending |
RedHill Biopharma, Ltd. 2019-04-18 |
 TALICIA 87235778 5392689 Live/Registered |
RedHill Biopharma, Ltd. 2016-11-14 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.