DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE, AND AMPHETAMINE SULFATE EXTENDED-RELEASE- dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate capsule, extended release
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate Extended-Release by
Drug Labeling and Warnings
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate Extended-Release by is a Prescription medication manufactured, distributed, or labeled by Rhodes Pharmaceuticals L.P.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE and AMPHETAMINE SULFATE extended-release capsules safely and effectively. See full prescribing information for dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules.
DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE and AMPHETAMINE SULFATE extended-release capsules, for oral use.
CII
Initial U.S. Approval: 2001WARNING: ABUSE AND DEPENDENCE
See full prescribing information for complete boxed warning
- CNS stimulants, including dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, other amphetamine-containing products, and methylphenidate, have a high potential for abuse and dependence (5.1, 9.3)
- Assess the risk of abuse prior to prescribing and monitor for signs of abuse and dependence while on therapy (9.2, 9.3).
RECENT MAJOR CHANGES
Boxed Warning 7/2019 Dosage and Administration (2.1) 7/2019 Warnings and Precautions (5.1) 7/2019 INDICATIONS AND USAGE
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, a CNS stimulant, are indicated for the treatment of attention deficit hyperactivity disorder (ADHD). (1)
- Children (ages 6 to 12): Efficacy was established in one 3-week outpatient, controlled trial and one analogue classroom, controlled trial in children with ADHD. (14)
- Adolescents (ages 13 to 17): Efficacy was established in one 4-week controlled trial in adolescents with ADHD. (14)
- Adults: Efficacy was established in one 4-week controlled trial in adults with ADHD. (14)
DOSAGE AND ADMINISTRATION
- Pediatric patients (ages 6 to 17): 10 mg once daily in the morning. Maximum dose for children 6 to 12 is 30 mg once daily. (2.2, 2.3, 2.4)
- Adults: 20 mg once daily in the morning. (2.5)
- Pediatric patients (ages 6 to 17) with severe renal impairment: 5 mg once daily in the morning. Maximum dose for children 6 to 12 years of age with severe renal impairment is 20 mg once daily. (2.6, 8.6)
- Adults with severe renal impairment: 15 mg once daily in the morning. (2.6, 8.6)
- Patients with ESRD: Not recommended. (2.6, 8.6)
DOSAGE FORMS AND STRENGTHS
- Extended release capsules: 5 mg, 10 mg, 15 mg, 20 mg, 25 mg, 30 mg (3)
CONTRAINDICATIONS
- Advanced arteriosclerosis (4)
- Symptomatic cardiovascular disease (4)
- Moderate to severe hypertension (4)
- Hyperthyroidism (4)
- Known hypersensitivity or idiosyncrasy to amphetamine (4)
- Glaucoma (4)
- Agitated states (4)
- History of drug abuse (4)
- During or within 14 days following the administration of monoamine oxidase inhibitors (MAOI) (4, 7.1)
WARNINGS AND PRECAUTIONS
- Serious Cardiovascular Events: Sudden death has been reported with usual doses of CNS stimulants in children and adolescents with structural cardiac abnormalities or other serious heart problems; sudden death, stroke, and myocardial infarction have been reported in adults taking CNS stimulants at usual doses. Stimulant drugs should not be used in patients with known structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other serious heart problems. (5.1)
- Increase in Blood Pressure: Monitor blood pressure and pulse at appropriate intervals. Use with caution in patients for whom blood pressure increases may be problematic. (5.2)
- Psychiatric Adverse Events: Stimulants may cause treatment-emergent psychotic or manic symptoms in patients with no prior history, or exacerbation of symptoms in patients with pre-existing psychosis. Evaluate for bipolar disorder prior to stimulant use. Monitor for aggressive behavior. (5.3)
- Long-term Suppression of Growth: Monitor height and weight at appropriate intervals. (5.4)
- Seizures: May lower the convulsive threshold. Discontinue in the presence of seizures. (5.5)
- Peripheral Vasculopathy, including Raynaud's phenomenon: Stimulants used to treat ADHD are associated with peripheral vasculopathy, including Raynaud's phenomenon. Careful observation for digital changes is necessary during treatment with ADHD stimulants. (5.6)
- Serotonin Syndrome: Increased risk when co-administered with serotonergic agents (e.g., SSRIs, SNRIs, triptans), but also during overdosage situations. If it occurs, discontinue dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules and initiate supportive treatment (4, 5.7, 10).
- Visual Disturbance: Difficulties with accommodation and blurring of vision have been reported with stimulant treatment. (5.8)
- Tics: May exacerbate tics. Evaluate for tics and Tourette's syndrome prior to stimulant administration. (5.9)
ADVERSE REACTIONS
- Children (ages 6 to 12): Most common adverse reactions (≥5% and with a higher incidence than on placebo) were loss of appetite, insomnia, abdominal pain, emotional lability, vomiting, nervousness, nausea, and fever. (6.1)
- Adolescents (ages 13 to 17): Most common adverse reactions (≥5% and with a higher incidence than on placebo) were loss of appetite, insomnia, abdominal pain, weight loss, and nervousness. (6.1)
- Adults: Most common adverse reactions (≥5% and with a higher incidence than on placebo) were dry mouth, loss of appetite, insomnia, headache, weight loss, nausea, anxiety, agitation, dizziness, tachycardia, diarrhea, asthenia, and urinary tract infections. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Rhodes Pharmaceuticals L.P. at 1-888-827-0616; or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- MAOI antidepressants are contraindicated; MAOIs potentiate the effects of amphetamine. Do not administer dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules during or within 14 days after use of MAOI. (4, 7.1)
- Alkalinizing agents (GI antacids and urinary): These agents increase blood levels of amphetamine. (7.1)
- Acidifying agents (GI and urinary): These agents reduce blood levels of amphetamine. (7.1)
- Adrenergic blockers, antihistamines, antihypertensives, phenobarbital, phenytoin, veratrum alkaloids, and ethosuximide: Effects may be reduced by amphetamines. (7.1)
- Tricyclic antidepressants, norepinephrine, and meperidine: Effects may be potentiated by amphetamines. (7.1)
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: ABUSE AND DEPENDENCE
1 INDICATIONS AND USAGE
1.1 Attention Deficit Hyperactivity Disorder
2 DOSAGE and ADMINISTRATION
2.1 Important Information Prior to Initiating Treatment
2.2 Dosing Considerations for All Patients
2.3 Children
2.4 Adolescents
2.5 Adults
2.6 Dosage in Patients with Renal Impairment
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Abuse and Dependence
5.2 Serious Cardiovascular Events
5.3 Psychiatric Adverse Events
5.4 Long-Term Suppression of Growth
5.5 Seizures
5.6 Peripheral Vasculopathy, including Raynaud's Phenomenon
5.7 Serotonin Syndrome
5.8 Visual Disturbance
5.9 Tics
5.10 Prescribing and Dispensing
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Adverse Reactions Associated with the Use of Amphetamine; Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate Extended-Release Capsules; or Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate Tablets (immediate-release)
7 DRUG INTERACTIONS
7.1 Clinically Important Interactions with Amphetamines
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: ABUSE AND DEPENDENCE
CNS stimulants, including dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, other amphetamine-containing products, and methylphenidate, have a high potential for abuse and dependence. Assess the risk of abuse prior to prescribing and monitor for signs of abuse and dependence while on therapy [see Warnings and Precautions (5.1) and Drug Abuse and Dependence (9.2, 9.3)].
-
1 INDICATIONS AND USAGE
1.1 Attention Deficit Hyperactivity Disorder
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules are indicated for the treatment of attention deficit hyperactivity disorder (ADHD).
The efficacy of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules in the treatment of ADHD was established on the basis of two controlled trials in children aged 6 to 12, one controlled trial in adolescents aged 13 to 17, and one controlled trial in adults who met DSM-IV® criteria for ADHD [see Clinical Studies (14)].
A diagnosis of ADHD (DSM-IV®) implies the presence of hyperactive-impulsive or inattentive symptoms that caused impairment and were present before age 7 years. The symptoms must cause clinically significant impairment, e.g., in social, academic, or occupational functioning, and be present in two or more settings, e.g., school (or work) and at home. The symptoms must not be better accounted for by another mental disorder. For the Inattentive Type, at least six of the following symptoms must have persisted for at least 6 months: lack of attention to details/careless mistakes; lack of sustained attention; poor listener; failure to follow through on tasks; poor organization; avoids tasks requiring sustained mental effort; loses things; easily distracted; forgetful. For the Hyperactive-Impulsive Type, at least six of the following symptoms must have persisted for at least 6 months: fidgeting/squirming; leaving seat; inappropriate running/climbing; difficulty with quiet activities; "on the go;" excessive talking; blurting answers; can't wait turn; intrusive. The Combined Type requires both inattentive and hyperactive-impulsive criteria to be met.
Special Diagnostic Considerations
Specific etiology of this syndrome is unknown and there is no single diagnostic test. Adequate diagnosis requires the use not only of medical but of special psychological, educational, and social resources. Learning may or may not be impaired. The diagnosis must be based upon a complete history and evaluation of the patient and not solely on the presence of the required number of DSM-IV® characteristics.
Need for Comprehensive Treatment Program
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules are indicated as an integral part of a total treatment program for ADHD that may include other measures (psychological, educational, social) for patients with this syndrome. Drug treatment may not be indicated for all patients with this syndrome. Stimulants are not intended for use in the patient who exhibits symptoms secondary to environmental factors and/or other primary psychiatric disorders, including psychosis. Appropriate educational placement is essential and psychosocial intervention is often helpful. When remedial measures alone are insufficient, the decision to prescribe stimulant medication will depend upon the physician's assessment of the chronicity and severity of the child's symptoms.
Long-Term Use
The effectiveness of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules for long-term use, i.e., for more than 3 weeks in children and 4 weeks in adolescents and adults, has not been systematically evaluated in controlled trials. Therefore, the physician who elects to use dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules for extended periods should periodically re-evaluate the long-term usefulness of the drug for the individual patient.
-
2 DOSAGE and ADMINISTRATION
2.1 Important Information Prior to Initiating Treatment
Prior to initiating treatment with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, assess for the presence of cardiac disease (e.g., perform a careful history, family history of sudden death or ventricular arrhythmia, and physical exam) [see Warnings and Precautions (5.2)].
Assess the risk of abuse prior to prescribing and monitor for signs of abuse and dependence while on therapy. Maintain careful prescription records, educate patients about abuse, monitor for signs for abuse and overdose, and periodically re-evaluate the need for dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules use [see Warnings and Precautions (5.1), Drug Abuse and Dependence (9)].
2.2 Dosing Considerations for All Patients
Individualize the dosage according to the therapeutic needs and response of the patient. Administer dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules at the lowest effective dosage.
Based on bioequivalence data, patients taking divided doses of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate tablets (immediate-release), (for example, twice daily), may be switched to dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules at the same total daily dose taken once daily. Titrate at weekly intervals to appropriate efficacy and tolerability as indicated.
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules may be taken whole, or the capsule may be opened and the entire contents sprinkled on applesauce. If the patient is using the sprinkle administration method, the sprinkled applesauce should be consumed immediately; it should not be stored. Patients should take the applesauce with sprinkled beads in its entirety without chewing. The dose of a single capsule should not be divided. The contents of the entire capsule should be taken, and patients should not take anything less than one capsule per day.
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules may be taken with or without food.
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules should be given upon awakening. Afternoon doses should be avoided because of the potential for insomnia.
Where possible, dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules therapy should be interrupted occasionally to determine if there is a recurrence of behavioral symptoms sufficient to require continued therapy.
2.3 Children
In children with ADHD who are 6 to 12 years of age and are either starting treatment for the first time or switching from another medication, start with 10 mg once daily in the morning; daily dosage may be adjusted in increments of 5 mg or 10 mg at weekly intervals. When in the judgment of the clinician a lower initial dose is appropriate, patients may begin treatment with 5 mg once daily in the morning. The maximum recommended dose for children 6 to 12 years of age is 30 mg/day; doses greater than 30 mg/day have not been studied in children. Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules have not been studied in children under 6 years of age.
2.4 Adolescents
The recommended starting dose for adolescents with ADHD who are 13 to17 years of age and are either starting treatment for the first time or switching from another medication is 10 mg/day. The dose may be increased to 20 mg/day after one week if ADHD symptoms are not adequately controlled.
2.5 Adults
In adults with ADHD who are either starting treatment for the first time or switching from another medication, the recommended dose is 20 mg/day.
2.6 Dosage in Patients with Renal Impairment
In adult patients with severe renal impairment (GFR 15 to < 30 mL/min/1.73m2), the recommended dose is 15 mg once daily in the morning. In pediatric patients (6 to 17 years of age) with severe renal impairment, the recommended dose is 5 mg once daily. The maximum dose for children 6 to 12 years of age with severe renal impairment is 20 mg once daily. Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules are not recommended in patients with end stage renal disease (ESRD) (GFR < 15 mL/min/1.73m2 [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHS
5 mg Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules: Blue Opaque Cap/Beige Opaque Body Hard Gelatin Capsule. Printed Laterally with "RP" on the cap and "705" on the body in black ink containing white to off-white beads. 10 mg Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules: Blue Opaque Cap/Blue Opaque Body Hard Gelatin Capsule. Printed Laterally with "RP" on the cap and "710" on the body in black ink containing white to off-white beads. 15 mg Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules: White Opaque Cap/Blue Opaque Body Hard Gelatin Capsule. Printed Laterally with "RP" on the cap and "715" on the body in black ink containing white to off-white beads. 20 mg Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules: Orange Opaque Cap/ Orange Opaque Body Hard Gelatin Capsule. Printed Laterally with "RP" on the cap and "720" on the body in black ink containing white to off-white beads. 25 mg Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules: White Opaque Cap/Orange Opaque Body Hard Gelatin Capsule. Printed Laterally with "RP" on the cap and "725" on the body in black ink containing white to off-white beads. 30 mg Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules: Orange Opaque Cap/Beige Opaque Body Hard Gelatin Capsule. Printed Laterally with "RP" on the cap and "730" on the body in black ink containing white to off-white beads. -
4 CONTRAINDICATIONS
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules administration is contraindicated in patients with the following conditions:
- Advanced arteriosclerosis
- Symptomatic cardiovascular disease
- Moderate to severe hypertension
- Hyperthyroidism
- In patients known to be hypersensitive to amphetamine, or other components of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules. Hypersensitivity reactions such as angioedema and anaphylactic reactions have been reported in patients treated with other amphetamine products [see Adverse Reactions (6.2)].
- Glaucoma
- Agitated states
- History of drug abuse
- Patients taking monoamine oxidase inhibitors (MAOIs), or within 14 days of stopping MAOIs (including MAOIs such as linezolid or intravenous methylene blue), because of an increased risk of hypertensive crisis [see Warnings and Precautions (5.6) and Drug Interactions (7.1)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Potential for Abuse and Dependence
CNS stimulants, including dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, other amphetamine-containing products, and methylphenidate, have a high potential for abuse and dependence. Assess the risk of abuse prior to prescribing, and monitor for signs of abuse and dependence while on therapy [see Boxed Warning, Drug Abuse and Dependence (9.2, 9.3)].
5.2 Serious Cardiovascular Events
Sudden Death and Pre-existing Structural Cardiac Abnormalities or Other Serious Heart Problems
Children and Adolescents
Sudden death has been reported in association with CNS stimulant treatment at usual doses in children and adolescents with structural cardiac abnormalities or other serious heart problems. Although some serious heart problems alone carry an increased risk of sudden death, stimulant products generally should not be used in children or adolescents with known serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, or other serious cardiac problems that may place them at increased vulnerability to the sympathomimetic effects of a stimulant drug [see Contraindications (4)].
Adults
Sudden deaths, stroke, and myocardial infarction have been reported in adults taking stimulant drugs at usual doses for ADHD. Although the role of stimulants in these adult cases is also unknown, adults have a greater likelihood than children of having serious structural cardiac abnormalities, cardiomyopathy, serious heart rhythm abnormalities, coronary artery disease, or other serious cardiac problems. Adults with such abnormalities should also generally not be treated with stimulant drugs [see Contraindications (4)].
Hypertension and Other Cardiovascular Conditions
Stimulant medications cause a modest increase in average blood pressure (about 2 to 4 mmHg) and average heart rate (about 3 to 6 bpm), and individuals may have larger increases. While the mean changes alone would not be expected to have short-term consequences, all patients should be monitored for larger changes in heart rate and blood pressure. Caution is indicated in treating patients whose underlying medical conditions might be compromised by increases in blood pressure or heart rate, e.g., those with pre-existing hypertension, heart failure, recent myocardial infarction, or ventricular arrhythmia [see Contraindications (4) and Adverse Reactions (6)].
Assessing Cardiovascular Status in Patients Being Treated with Stimulant Medications
Children, adolescents, or adults who are being considered for treatment with stimulant medications should have a careful history (including assessment for a family history of sudden death or ventricular arrhythmia) and physical exam to assess for the presence of cardiac disease, and should receive further cardiac evaluation if findings suggest such disease (e.g. electrocardiogram and echocardiogram). Patients who develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease during stimulant treatment should undergo a prompt cardiac evaluation.
5.3 Psychiatric Adverse Events
Pre-Existing Psychosis
Administration of stimulants may exacerbate symptoms of behavior disturbance and thought disorder in patients with pre-existing psychotic disorder.
Bipolar Illness
Particular care should be taken in using stimulants to treat ADHD patients with comorbid bipolar disorder because of concern for possible induction of mixed/manic episode in such patients. Prior to initiating treatment with a stimulant, patients with comorbid depressive symptoms should be adequately screened to determine if they are at risk for bipolar disorder; such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and depression.
Emergence of New Psychotic or Manic Symptoms
Treatment-emergent psychotic or manic symptoms, e.g., hallucinations, delusional thinking, or mania in children and adolescents without prior history of psychotic illness or mania can be caused by stimulants at usual doses. If such symptoms occur, consideration should be given to a possible causal role of the stimulant, and discontinuation of treatment may be appropriate. In a pooled analysis of multiple short-term, placebo-controlled studies, such symptoms occurred in about 0.1% (4 patients with events out of 3482 exposed to methylphenidate or amphetamine for several weeks at usual doses) of stimulant-treated patients compared to 0 in placebo-treated patients.
Aggression
Aggressive behavior or hostility is often observed in children and adolescents with ADHD, and has been reported in clinical trials and the postmarketing experience of some medications indicated for the treatment of ADHD. Although there is no systematic evidence that stimulants cause aggressive behavior or hostility, patients beginning treatment for ADHD should be monitored for the appearance of or worsening of aggressive behavior or hostility.
5.4 Long-Term Suppression of Growth
Monitor growth in children during treatment with stimulants. Patients who are not growing or gaining weight as expected may need to have their treatment interrupted.
Careful follow-up of weight and height in children ages 7 to 10 years who were randomized to either methylphenidate or non-medication treatment groups over 14 months, as well as in naturalistic subgroups of newly methylphenidate-treated and non-medication treated children over 36 months (to the ages of 10 to 13 years), suggests that consistently medicated children (i.e., treatment for 7 days per week throughout the year) have a temporary slowing in growth rate (on average, a total of about 2 cm less growth in height and 2.7 kg less growth in weight over 3 years), without evidence of growth rebound during this period of development.
In a controlled trial of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules in adolescents, mean weight change from baseline within the initial 4 weeks of therapy was -1.1 lbs. and -2.8 lbs., respectively, for patients receiving 10 mg and 20 mg dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules. Higher doses were associated with greater weight loss within the initial 4 weeks of treatment. Chronic use of amphetamines can be expected to cause a similar suppression of growth.
5.5 Seizures
There is some clinical evidence that stimulants may lower the convulsive threshold in patients with prior history of seizures, in patients with prior EEG abnormalities in the absence of seizures, and very rarely, in patients without a history of seizures and no prior EEG evidence of seizures. In the presence of seizures, dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules should be discontinued.
5.6 Peripheral Vasculopathy, including Raynaud's Phenomenon
Stimulants, including dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, used to treat ADHD are associated with peripheral vasculopathy, including Raynaud's phenomenon. Signs and symptoms are usually intermittent and mild; however, very rare sequelae include digital ulceration and/or soft tissue breakdown. Effects of peripheral vasculopathy, including Raynaud's phenomenon, were observed in post-marketing reports at different times and at therapeutic doses in all age groups throughout the course of treatment. Signs and symptoms generally improve after reduction in dose or discontinuation of drug. Careful observation for digital changes is necessary during treatment with ADHD stimulants. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients.
5.7 Serotonin Syndrome
Serotonin syndrome, a potentially life-threatening reaction, may occur when amphetamines are used in combination with other drugs that affect the serotonergic neurotransmitter systems such as MAOIs, selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John's Wort [see Drug Interactions (7.1)]. Amphetamines and amphetamine derivatives are known to be metabolized, to some degree, by cytochrome P450 2D6 (CYP2D6) and display minor inhibition of CYP2D6 metabolism [see Clinical Pharmacology (12.3)]. The potential for a pharmacokinetic interaction exists with the co-administration of CYP2D6 inhibitors which may increase the risk with increased exposure to dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules. In these situations, consider an alternative non-serotonergic drug or an alternative drug that does not inhibit CYP2D6 [see Drug Interactions (7.1)]. Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
Concomitant use of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules with MAOI drugs is contraindicated [see Contraindications (4)].
Discontinue treatment with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules and any concomitant serotonergic agents immediately if symptoms of serotonin syndrome occur, and initiate supportive symptomatic treatment. Concomitant use of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules with other serotonergic drugs or CYP2D6 inhibitors should be used only if the potential benefit justifies the potential risk. If clinically warranted, consider initiating dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules with lower doses, monitoring patients for the emergence of serotonin syndrome during drug initiation or titration, and informing patients of the increased risk for serotonin syndrome.
5.8 Visual Disturbance
Difficulties with accommodation and blurring of vision have been reported with stimulant treatment.
5.9 Tics
Amphetamines have been reported to exacerbate motor and phonic tics and Tourette's syndrome. Therefore, clinical evaluation for tics and Tourette's syndrome in patients and their families should precede use of stimulant medications.
5.10 Prescribing and Dispensing
The least amount of amphetamine feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage. Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules should be used with caution in patients who use other sympathomimetic drugs.
-
6 ADVERSE REACTIONS
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.1 Clinical Trials Experience
The premarketing development program for dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules included exposures in a total of 1315 participants in clinical trials (635 pediatric patients, 350 adolescent patients, 248 adult patients, and 82 healthy adult subjects). Of these, 635 patients (ages 6 to 12) were evaluated in two controlled clinical studies, one open-label clinical study, and two single-dose clinical pharmacology studies (N= 40). Safety data on all patients are included in the discussion that follows. Adverse reactions were assessed by collecting adverse reactions, results of physical examinations, vital signs, weights, laboratory analyses, and ECGs.
Adverse reactions during exposure were obtained primarily by general inquiry and recorded by clinical investigators using terminology of their own choosing. Consequently, it is not possible to provide a meaningful estimate of the proportion of individuals experiencing adverse reactions without first grouping similar types of reactions into a smaller number of standardized event categories. In the tables and listings that follow, COSTART terminology has been used to classify reported adverse reactions.
The stated frequencies of adverse reactions represent the proportion of individuals who experienced, at least once, a treatment-emergent adverse event of the type listed.
Adverse Reactions Leading to Discontinuation of Treatment
In two placebo-controlled studies of up to 5 weeks duration among children with ADHD, 2.4% (10/425) of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules-treated patients discontinued due to adverse reactions (including 3 patients with loss of appetite, one of whom also reported insomnia) compared to 2.7% (7/259) receiving placebo.
The most frequent adverse reactions leading to discontinuation of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules in controlled and uncontrolled, multiple-dose clinical trials of children (N=595) were anorexia (loss of appetite) (2.9%), insomnia (1.5%), weight loss (1.2%), emotional lability (1%), and depression (0.7%). Over half of these patients were exposed to dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules for 12 months or more.
In a separate placebo-controlled 4-week study in adolescents with ADHD, five patients (2.1%) discontinued treatment due to adverse events among dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules-treated patients (N=233) compared to none who received placebo (N=54). The most frequent adverse event leading to discontinuation and considered to be drug-related (i.e. leading to discontinuation in at least 1% of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules-treated patients and at a rate at least twice that of placebo) was insomnia (1.3%, n=3).
In one placebo-controlled 4-week study among adults with ADHD with doses 20 mg to 60 mg, 23 patients (12.0%) discontinued treatment due to adverse events among dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules-treated patients (N=191) compared to one patient (1.6%) who received placebo (N=64). The most frequent adverse events leading to discontinuation and considered to be drug-related (i.e. leading to discontinuation in at least 1% of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules-treated patients and at a rate at least twice that of placebo) were insomnia (5.2%, n=10), anxiety (2.1%, n=4), nervousness (1.6%, n=3), dry mouth (1.6%, n=3), anorexia (1.6%, n=3), tachycardia (1.6%, n=3), headache (1.6%, n=3), and asthenia (1.0%, n=2).
Adverse Reactions Occurring in Controlled Trials
Adverse reactions reported in a 3-week clinical trial of children and a 4-week clinical trial in adolescents and adults, respectively, treated with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules or placebo are presented in the tables below.
Table 1 Adverse Reactions Reported by 2% or More of Children (6 to12 Years Old) Receiving Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate Extended-Release Capsules with Higher Incidence Than on Placebo in a 584-Patient Clinical Study Body System Preferred Term Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules
(n=374)Placebo
(n=210)General Abdominal Pain (stomachache) 14% 10% Fever 5% 2% Infection 4% 2% Accidental Injury 3% 2% Asthenia (fatigue) 2% 0% Digestive System Loss of Appetite 22% 2% Vomiting 7% 4% Nausea 5% 3% Dyspepsia 2% 1% Nervous System Insomnia 17% 2% Emotional Lability 9% 2% Nervousness 6% 2% Dizziness 2% 0% Metabolic/Nutritional Weight Loss 4% 0% Table 2 Adverse Reactions Reported by 5% or More of Adolescents (13 to 17 Years Old) Weighing ≤ 75 kg/165 lbs Receiving Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate Extended-Release Capsules with Higher Incidence Than Placebo in a 287 Patient Clinical Forced Weekly-Dose Titration Study* Body System Preferred Term Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules (n=233) Placebo
(n=54)Note: The following reactions did not meet the criterion for inclusion in Table 2 but were reported by 2% to 4% of adolescent patients receiving Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules with a higher incidence than patients receiving placebo in this study: accidental injury, asthenia (fatigue), dry mouth, dyspepsia, emotional lability, nausea, somnolence, and vomiting. - * Included doses up to 40 mg
- † Dose-related adverse reactions
- ‡ Appears the same due to rounding
General Abdominal Pain (stomachache) 11% 2% Digestive System Loss of Appetite† 36% 2% Nervous System Insomnia†
Nervousness12%
6%4%
6%‡Metabolic/Nutritional Weight Loss† 9% 0% Table 3 Adverse Reactions Reported by 5% or More of Adults Receiving Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate Extended-Release Capsules with Higher Incidence Than on Placebo in a 255 Patient Clinical Forced Weekly-Dose Titration Study* Body System Preferred Term Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules (n=191) Placebo (n=64) Note: The following reactions did not meet the criterion for inclusion in Table 3 but were reported by 2% to 4% of adult patients receiving dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules with a higher incidence than patients receiving placebo in this study: infection, photosensitivity reaction, constipation, tooth disorder (e.g., teeth clenching, tooth infection), emotional lability, libido decreased, somnolence, speech disorder (e.g., stuttering, excessive speech), palpitation, twitching, dyspnea, sweating, dysmenorrhea, and impotence. - * Included doses up to 60 mg.
- † Appears the same due to rounding
General Headache
Asthenia26%
6%13%
5%Digestive System Dry Mouth
Loss of Appetite
Nausea
Diarrhea35%
33%
8%
6%5%
3%
3%
0%Nervous System Insomnia
Agitation
Anxiety
Dizziness
Nervousness27%
8%
8%
7%
13%13%
5%
5%
0%
13%†Cardiovascular System Tachycardia 6% 3% Metabolic/Nutritional Weight Loss 10% 0% Urogenital System Urinary Tract Infection 5% 0% Hypertension [see Warnings and Precautions (5.1)]
In a controlled 4-week outpatient clinical study of adolescents with ADHD, isolated systolic blood pressure elevations ≥15 mmHg were observed in 7/64 (11%) placebo-treated patients and 7/100 (7%) patients receiving dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules 10 or 20 mg. Isolated elevations in diastolic blood pressure ≥ 8 mmHg were observed in 16/64 (25%) placebo-treated patients and 22/100 (22%) dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules-treated patients. Similar results were observed at higher doses.
In a single-dose pharmacokinetic study in 23 adolescents with ADHD, isolated increases in systolic blood pressure (above the upper 95% CI for age, gender, and stature) were observed in 2/17 (12%) and 8/23 (35%), subjects administered 10 mg and 20 mg dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, respectively. Higher single doses were associated with a greater increase in systolic blood pressure. All increases were transient, appeared maximal at 2 to 4 hours post dose and not associated with symptoms.
6.2 Adverse Reactions Associated with the Use of Amphetamine; Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate Extended-Release Capsules; or Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate Tablets (immediate-release)
The following adverse reactions have been identified during post-approval use of amphetamine; dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules; or dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate tablets (immediate-release). Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiovascular
Palpitations. There have been isolated reports of cardiomyopathy associated with chronic amphetamine use.
Central Nervous System
Psychotic episodes at recommended doses, overstimulation, restlessness, irritability, euphoria, dyskinesia, dysphoria, depression, tremor, tics, aggression, anger, logorrhea, dermatillomania, paresthesia (including formication), and bruxism.
-
7 DRUG INTERACTIONS
7.1 Clinically Important Interactions with Amphetamines
Table 4: Drugs Having Clinically Important Interactions with Amphetamines Monoamine Oxidase Inhibitors (MAOIs) Clinical Impact Concomitant use of MAOIs and CNS stimulants can cause hypertensive crisis. Potential outcomes include death, stroke, myocardial infarction, aortic dissection, ophthalmological complications, eclampsia, pulmonary edema, and renal failure. Intervention Do not administer dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules concomitantly or within 14 days after discontinuing MAOI [see Contraindications (4). Examples selegiline, tranylcypromine, isocarboxazid, phenelzine, linezolid, methylene blue Serotonergic Drugs Clinical Impact The concomitant use of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules and serotonergic drugs increases the risk of serotonin syndrome. Intervention Initiate with lower doses and monitor patients for signs and symptoms of serotonin syndrome, particularly during dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules initiation or dosage increase. If serotonin syndrome occurs, discontinue dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules and the concomitant serotonergic drug(s) [see Warnings and Precautions (5.6)]. Examples selective serotonin reuptake inhibitors (SSRI), serotonin norepinephrine reuptake inhibitors (SNRI), triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, St. John's Wort CYP2D6 Inhibitors Clinical Impact The concomitant use of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules and CYP2D6 inhibitors may increase the exposure of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules compared to the use of the drug alone and increase the risk of serotonin syndrome. Intervention Initiate with lower doses and monitor patients for signs and symptoms of serotonin syndrome particularly during dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules initiation and after a dosage increase. If serotonin syndrome occurs, discontinue dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules and the CYP2D6 inhibitor [see Warnings and Precautions (5.6) and Overdosage (10)]. Examples paroxetine and fluoxetine (also serotonergic drugs), quinidine, ritonavir Alkalinizing Agents Clinical Impact Increase blood levels and potentiate the action of amphetamine. Intervention Co-administration of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules and gastrointestinal or urinary alkalinizing agents should be avoided. Examples Gastrointestinal alkalinizing agents (e.g., sodium bicarbonate). Urinary alkalinizing agents (e.g. acetazolamide, some thiazides). Acidifying Agents Clinical Impact Lower blood levels and efficacy of amphetamines. Intervention Increase dose based on clinical response. Examples Gastrointestinal acidifying agents (e.g., guanethidine, reserpine, glutamic acid HCl, ascorbic acid).
Urinary acidifying agents (e.g., ammonium chloride, sodium acid phosphate, methenamine salts).Tricyclic Antidepressants Clinical Impact May enhance the activity of tricyclic or sympathomimetic agents causing striking and sustained increases in the concentration of d-amphetamine in the brain; cardiovascular effects can be potentiated. Intervention Monitor frequently and adjust or use alternative therapy based on clinical response. Examples desipramine, protriptyline Proton Pump Inhibitors Clinical Impact Time to maximum concentration (Tmax) of amphetamine is decreased compared to when administered alone. Intervention Monitor patients for changes in clinical effect and adjust therapy based on clinical response. Examples Omeprazole -
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychostimulants at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/clinical-and-researchprograms/pregnancyregistry/othermedications/.
Risk Summary
Available data from published epidemiologic studies and postmarketing reports on use of prescription amphetamine in pregnant women have not identified a drug-associated risk of major birth defects and miscarriage (see Data). Adverse pregnancy outcomes, including premature delivery and low birth weight, have been seen in infants born to mothers taking amphetamines during pregnancy (see Clinical Considerations).
No apparent effects on morphological development were observed in embryo-fetal development studies, with oral administration of amphetamine to rats and rabbits during organogenesis at doses 2 and 12 times, respectively, the maximum recommended human dose (MRHD) of 20 mg/day given to adolescents, on a mg/m2 basis. However, in a pre- and post-natal development study, amphetamine (d- to l- ratio of 3:1) administered orally to pregnant rats during gestation and lactation caused a decrease in pup survival and a decrease in pup body weight that correlated with a delay in developmental landmarks at clinically relevant doses of amphetamine. In addition, adverse effects on reproductive performance were observed in pups whose mothers were treated with amphetamine. Long-term neurochemical and behavioral effects have also been reported in animal developmental studies using clinically relevant doses of amphetamine (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15- 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Amphetamines, such as dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, cause vasoconstriction and thereby may decrease placental perfusion. In addition, amphetamines can stimulate uterine contractions, increasing the risk of premature delivery. Infants born to mothers taking amphetamines during pregnancy have an increased risk of premature delivery and low birth weight.
Monitor infants born to mothers taking amphetamines for symptoms of withdrawal such as feeding difficulties, irritability, agitation, and excessive drowsiness.
Data
Animal Data
Amphetamine (d- to l- enantiomer ratio of 3:1) had no apparent effects on embryofetal morphological development or survival when administered orally to pregnant rats and rabbits throughout the period of organogenesis at doses of up to 6 and 16 mg/kg/day, respectively. These doses are approximately 2 and 12 times, respectively, the maximum recommended human dose (MRHD) of 20 mg/day given to adolescents, on a mg/m2 basis. Fetal malformations and death have been reported in mice following parenteral administration of d-amphetamine doses of 50 mg/kg/day (approximately 10 times the MRHD given to adolescents on a mg/m2 basis) or greater to pregnant animals. Administration of these doses was also associated with severe maternal toxicity.
A study was conducted in which pregnant rats received daily oral doses of amphetamine (d- to l-enantiomer ratio of 3:1) of 2, 6, and 10 mg/kg from gestation day 6 to lactation day 20. These doses are approximately 0.8, 2, and 4 times the MRHD of 20 mg/day given to adolescents, on a mg/m2 basis. All doses caused hyperactivity and decreased weight gain in the dams. A decrease in pup survival was seen at all doses. A decrease in pup body weight was seen at 6 and 10 mg/kg which correlated with delays in developmental landmarks, such as preputial separation and vaginal opening. Increased pup locomotor activity was seen at 10 mg/kg on day 22 postpartum but not at 5 weeks postweaning. When pups were tested for reproductive performance at maturation, gestational weight gain, number of implantations, and number of delivered pups were decreased in the group whose mothers had been given 10 mg/kg.
A number of studies from the literature in rodents indicate that prenatal or early postnatal exposure to amphetamine (d- or d, l-) at doses similar to those used clinically can result in long-term neurochemical and behavioral alterations. Reported behavioral effects include learning and memory deficits, altered locomotor activity, and changes in sexual function.
8.2 Lactation
Risk Summary
Based on limited case reports in published literature, amphetamine (d- or d, l-) is present in human milk, at relative infant doses of 2% to 13.8% of the maternal weight-adjusted dosage and a milk/plasma ratio ranging between 1.9 and 7.5. There are no reports of adverse effects on the breastfed infant. Long-term neurodevelopmental effects on infants from amphetamine exposure are unknown. It is possible that large dosages of amphetamine might interfere with milk production, especially in women whose lactation is not well established. Because of the potential for serious adverse reactions in nursing infants, advise patients that breastfeeding is not recommended during treatment with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules.
8.4 Pediatric Use
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules are indicated for use in children 6 years of age and older.
The safety and efficacy of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules in children under 6 years of age have not been studied. Long-term effects of amphetamines in children have not been well established.
Long-Term Growth Suppression
Growth should be monitored during treatment with stimulants, including dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, and pediatric patients aged 6 to 17 years who are not growing or gaining weight as expected may need to have their treatment interrupted [see Warnings and Precautions (5.3)].
Juvenile Animal Toxicity Data
Juvenile rats treated with mixed amphetamine salts early in the postnatal period through sexual maturation demonstrated transient changes in motor activity. Learning and memory was impaired at approximately 6 times the maximum recommended human dose (MRHD) given to children on a mg/m2 basis. No recovery was seen following a drug free period. A delay in sexual maturation was observed at a dose approximately 6 times the MRHD given to children on a mg/m2 basis, although there was no effect on fertility.
In a juvenile developmental study, rats received daily oral doses of amphetamine (d to l enantiomer ratio of 3:1), of 2, 6, or 20 mg/kg on days 7 to 13 of age; from day 14 to approximately day 60 of age these doses were given b.i.d. for total daily doses of 4, 12, or 40 mg/kg. The latter doses are approximately 0.6, 2, and 6 times the MRHD of 30 mg/day, given to children on a mg/m2 basis. Post dosing hyperactivity was seen at all doses; motor activity measured prior to the daily dose was decreased during the dosing period but the decreased motor activity was largely absent after an 18-day drug-free recovery period. Performance in the Morris water maze test for learning and memory was impaired at the 40 mg/kg dose, and sporadically at the lower doses, when measured prior to the daily dose during the treatment period; no recovery was seen after a 19-day drug-free period. A delay in the developmental milestones of vaginal opening and preputial separation was seen at 40 mg/kg but there was no effect on fertility.
8.5 Geriatric Use
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules have not been studied in the geriatric population.
8.6 Renal Impairment
Due to reduced clearance of amphetamines in patients with severe renal impairment (GFR 15 to <30 mL/min/1.73m2), the recommended dose should be reduced. Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules are not recommended in patients with ESRD (GFR < 15 ml/min/1.73m2) [see Dosage and Administration (2.5), Clinical Pharmacology (12.3)].
d-Amphetamine is not dialyzable.
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules contain amphetamine, a Schedule II controlled substance.
9.2 Abuse
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules are a CNS stimulant that contains amphetamine, which has a high potential for abuse. Abuse is characterized by impaired control of drug use, compulsive use despite harm, and craving.
Signs and symptoms of amphetamine abuse may include increased heart rate, respiratory rate, blood pressure, and/or sweating, dilated pupils, hyperactivity, restlessness, insomnia, decreased appetite, loss of coordination, tremors, flushed skin, vomiting, and/or abdominal pain. Anxiety, psychosis, hostility, aggression, suicidal or homicidal ideation have also been observed. Abusers of amphetamines may use other unapproved routes of administration which can result in overdose and death [see Overdosage (10)].
To reduce the abuse of CNS stimulants, including dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, assess the risk of abuse prior to prescribing. After prescribing, keep careful prescription records, educate patients and their families about abuse and proper storage and disposal of CNS stimulants. Monitor for signs of abuse while on therapy and re-evaluate the need for dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules use.
9.3 Dependence
Tolerance (a state of adaptation in which exposure to a specific dose of a drug results in a reduction of the drug's desired and/or undesired effects over time, in such a way that a higher dose of the drug is required to produce the same effect that was once obtained at a lower dose) may occur during chronic therapy of CNS stimulants including dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules.
Physical Dependence (which is manifested by a withdrawal syndrome produced by abrupt cessation, rapid dose reduction, or administration of an antagonist) may occur in patients treated with CNS stimulants including dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules. Withdrawal symptoms after abrupt cessation of CNS stimulants include dysphoric mood; fatigue; vivid, unpleasant dreams; insomnia or hypersomnia; increased appetite; and psychomotor retardation or agitation.
-
10 OVERDOSAGE
Manifestations of amphetamine overdose include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, panic states, hyperpyrexia, and rhabdomyolysis. Fatigue and depression usually follow the central nervous system stimulation. Serotonin syndrome has been reported with amphetamine use, including dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules. Cardiovascular effects include arrhythmias, hypertension or hypotension and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea and abdominal cramps. Fatal poisoning is usually preceded by convulsions and coma.
Treatment
Consult with a Certified Poison Control Center for up to date guidance and advice.
The prolonged release of mixed amphetamine salts from dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules should be considered when treating patients with overdose.
d-Amphetamine is not dialyzable.
-
11 DESCRIPTION
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules are a once daily extended-release, single-entity amphetamine product. Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules combine the neutral sulfate salts of dextroamphetamine and amphetamine, with the dextro isomer of amphetamine saccharate and d,l-amphetamine aspartate monohydrate. The dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended release capsules are a dose proportional composition using a multi-layer fluid bed bead coating process, followed by encapsulation using hard gelatin capsules. The dosage utilizes a bead that is coated with an extended-release drug layer, that is then coated with an enteric polymer to control drug release, and finally coated with an immediate-release drug layer.
Each capsule contains:
Capsule Strength5 mg 10 mg 15 mg 20 mg 25 mg 30 mg Dextroamphetamine Saccharate 1.25 mg 2.5 mg 3.75 mg 5.0 mg 6.25 mg 7.5 mg Amphetamine (D,L)-Aspartate Monohydrate 1.25 mg 2.5 mg 3.75 mg 5.0 mg 6.25 mg 7.5 mg Dextroamphetamine Sulfate, USP 1.25 mg 2.5 mg 3.75 mg 5.0 mg 6.25 mg 7.5 mg Amphetamine Sulfate, USP 1.25 mg 2.5 mg 3.75 mg 5.0 mg 6.25 mg 7.5 mg Total amphetamine base equivalence mg 3.1 mg 6.3 mg 9.4 mg 12.5 mg 15.6 mg 18.8 mg d-amphetamine base equivalence mg 2.4 mg 4.7 mg 7.1 mg 9.5 mg 11.9 mg 14.2 mg l-amphetamine base equivalence 0.75 mg 1.5 mg 2.3 mg 3.0 mg 3.8 mg 4.5 mg Inactive Ingredients and Colors
The inactive ingredients in dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules include: sugar spheres (sucrose, sugar syrup, and corn starch), hypromellose, talc, triethyl citrate, methacrylic acid copolymer dispersion and gelatin capsules. Gelatin capsules contain titanium dioxide and FD&C Blue# 1. The 5 mg, 20 mg, 25 mg, and 30 mg capsules also contain FD&C Yellow#6. The 5 mg, 10 mg, and 15 mg also contain D&C Yellow#10 and D&C Red#33. Additionally, the imprint ink contains the following non-volatile ingredients: shellac, black iron oxide and potassium hydroxide.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Amphetamines are non-catecholamine sympathomimetic amines with CNS stimulant activity. The mode of therapeutic action in ADHD is not known.
12.2 Pharmacodynamics
Amphetamines block the reuptake of norepinephrine and dopamine into the presynaptic neuron and increase the release of these monoamines into the extraneuronal space.
12.3 Pharmacokinetics
Pharmacokinetic studies of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules have been conducted in healthy adult and pediatric (children aged 6 to 12 yrs) subjects, and adolescent (13 to 17 yrs) and children with ADHD. Both dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate tablets (immediate-release) and dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules contain d-amphetamine and l-amphetamine salts in the ratio of 3:1. Following administration of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate tablets (immediate-release), the peak plasma concentrations occurred in about 3 hours for both d-amphetamine and l-amphetamine.
The time to reach maximum plasma concentration (Tmax) for dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules is about 7 hours, which is about 4 hours longer compared to dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate tablets (immediate-release). This is consistent with the extended-release nature of the product.
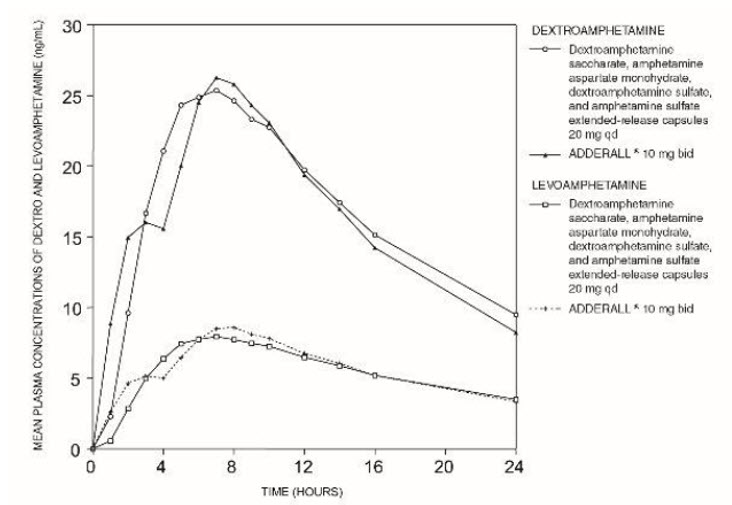
Figure 1: Mean d-amphetamine and l-amphetamine Plasma Concentrations Following Administration of Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate Extended-Release Capsules 20 mg (8 am) and Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate Tablets (immediate-release) 10 mg Twice Daily (8 am and 12 noon) in the Fed State.
A single dose of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended release 20 mg capsules provided comparable plasma concentration profiles of both d-amphetamine and l-amphetamine to dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate tablets (immediate-release) 10 mg twice daily administered 4 hours apart.
The mean elimination half-life for d-amphetamine is 10 hours in adults; 11 hours in adolescents aged 13 to 17 years and weighing less than or equal to 75 kg/165 lbs; and 9 hours in children aged 6 to 12 years. For the l-amphetamine, the mean elimination half-life in adults is 13 hours; 13 to 14 hours in adolescents; and 11 hours in children aged 6 to 12 years. On a mg/kg body weight basis, children have a higher clearance than adolescents or adults (see Special Populations below).
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules demonstrates linear pharmacokinetics over the dose range of 20 to 60 mg in adults and adolescents weighing greater than 75 kg/165 lbs, over the dose range of 10 to 40 mg in adolescents weighing less than or equal to 75 kg/165 lbs, and 5 to 30 mg in children aged 6 to 12 years. There is no unexpected accumulation at steady state in children.
Food does not affect the extent of absorption of d-amphetamine and l-amphetamine, but prolongs Tmax by 2.5 hours (from 5.2 hrs. at fasted state to 7.7 hrs. after a high-fat meal) for d-amphetamine and 2.7 hours (from 5.6 hrs. at fasted state to 8.3 hrs. after a high fat meal) for l-amphetamine after administration of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release, 30 mg capsule. Opening the capsule and sprinkling the contents on applesauce results in comparable absorption to the intact capsule taken in the fasted state. Equal doses of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules strengths are bioequivalent.
Metabolism and Excretion
Amphetamine is reported to be oxidized at the 4 position of the benzene ring to form 4-hydroxyamphetamine, or on the side chain α or β carbons to form alpha-hydroxy-amphetamine or norephedrine, respectively. Norephedrine and 4-hydroxy-amphetamine are both active and each is subsequently oxidized to form 4-hydroxy-norephedrine. Alpha-hydroxy-amphetamine undergoes deamination to form phenylacetone, which ultimately forms benzoic acid and its glucuronide and the glycine conjugate hippuric acid. Although the enzymes involved in amphetamine metabolism have not been clearly defined, CYP2D6 is known to be involved with formation of 4-hydroxy-amphetamine. Since CYP2D6 is genetically polymorphic, population variations in amphetamine metabolism are a possibility.
Amphetamine is known to inhibit monoamine oxidase, whereas the ability of amphetamine and its metabolites to inhibit various P450 isozymes and other enzymes has not been adequately elucidated. In vitro experiments with human microsomes indicate minor inhibition of CYP2D6 by amphetamine and minor inhibition of CYP1A2, 2D6, and 3A4 by one or more metabolites. However, due to the probability of auto-inhibition and the lack of information on the concentration of these metabolites relative to in vivo concentrations, no predications regarding the potential for amphetamine or its metabolites to inhibit the metabolism of other drugs by CYP isozymes in vivo can be made.
With normal urine pHs, approximately half of an administered dose of amphetamine is recoverable in urine as derivatives of alpha-hydroxy-amphetamine and approximately another 30 to 40% of the dose is recoverable in urine as amphetamine itself. Since amphetamine has a pKa of 9.9, urinary recovery of amphetamine is highly dependent on pH and urine flow rates. Alkaline urine pHs result in less ionization and reduced renal elimination, and acidic pHs and high flow rates result in increased renal elimination with clearances greater than glomerular filtration rates, indicating the involvement of active secretion. Urinary recovery of amphetamine has been reported to range from 1% to 75%, depending on urinary pH, with the remaining fraction of the dose hepatically metabolized. Consequently, both hepatic and renal dysfunction have the potential to inhibit the elimination of amphetamine and result in prolonged exposures. In addition, drugs that effect urinary pH are known to alter the elimination of amphetamine, and any decrease in amphetamine's metabolism that might occur due to drug interactions or genetic polymorphisms is more likely to be clinically significant when renal elimination is decreased [see Drug Interactions (7)].
Special Populations
Comparison of the pharmacokinetics of d- and l-amphetamine after oral administration of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules in children (6 to 12 years) and adolescent (13 to 17 years) ADHD patients and healthy adult volunteers indicates that body weight is the primary determinant of apparent differences in the pharmacokinetics of d- and l-amphetamine across the age range. Systemic exposure measured by area under the curve to infinity (AUC∞) and maximum plasma concentration (Cmax) decreased with increases in body weight, while oral volume of distribution (VZ/F), oral clearance (CL/F), and elimination half-life (t1/2) increased with increases in body weight.
Pediatric Patients
On a mg/kg weight basis, children eliminated amphetamine faster than adults. The elimination half-life (t1/2) is approximately 1 hour shorter for d-amphetamine and 2 hours shorter for l-amphetamine in children than in adults. However, children had higher systemic exposure to amphetamine (Cmax and AUC) than adults for a given dose of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, which was attributed to the higher dose administered to children on a mg/kg body weight basis compared to adults. Upon dose normalization on a mg/kg basis, children showed 30% less systemic exposure compared to adults.
Gender
Systemic exposure to amphetamine was 20 to 30% higher in women (N=20) than in men (N=20) due to the higher dose administered to women on a mg/kg body weight basis. When the exposure parameters (Cmax and AUC) were normalized by dose (mg/kg), these differences diminished. Age and gender had no direct effect on the pharmacokinetics of d- and l-amphetamine.
Race
Formal pharmacokinetic studies for race have not been conducted. However, amphetamine pharmacokinetics appeared to be comparable among Caucasians (N=33), Blacks (N=8) and Hispanics (N=10).
Patients with Renal Impairment
The effect of renal impairment on d- and l-amphetamine after administration of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules has not been studied. The impact of renal impairment on the disposition of amphetamine is expected to be similar between oral administration of lisdexamfetamine and dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules
In a pharmacokinetic study of lisdexamfetamine in adult subjects with normal and impaired renal function, mean d-amphetamine clearance was reduced from 0.7 L/hr/kg in normal subjects to 0.4 L/hr/kg in subjects with severe renal impairment (GFR 15 to <30mL/min/1.73m2). Dialysis did not significantly affect the clearance of d-amphetamine [see Use in Specific Populations (8.6)].
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No evidence of carcinogenicity was found in studies in which d,l-amphetamine (enantiomer ratio of 1:1) was administered to mice and rats in the diet for 2 years at doses of up to 30 mg/kg/day in male mice, 19 mg/kg/day in female mice, and 5 mg/kg/day in male and female rats. These doses are approximately 2.4, 1.5, and 0.8 times, respectively, the maximum recommended human dose of 30 mg/day given to children, on a mg/m2 basis.
Mutagenesis
Amphetamine, in the enantiomer ratio d- to l- ratio of 3:1), was not clastogenic in the mouse bone marrow micronucleus test in vivo and was negative when tested in the E. coli component of the Ames test in vitro. d,l-Amphetamine (1:1 enantiomer ratio) has been reported to produce a positive response in the mouse bone marrow micronucleus test, an equivocal response in the Ames test, and negative responses in the in vitro sister chromatid exchange and chromosomal aberration assays.
Impairment of Fertility
Amphetamine, in the enantiomer ratio d- to l- ratio of 3:1, did not adversely affect fertility or early embryonic development in the rat at doses of up to 20 mg/kg/day (approximately 8 times the maximum recommended human dose of 20 mg/day given to adolescents, on a mg/m2 basis).
-
14 CLINICAL STUDIES
Pediatric Patients
A double-blind, randomized, placebo-controlled, parallel-group study was conducted in children aged 6 to 12 (N=584) who met DSM-IV® criteria for ADHD (either the combined type or the hyperactive-impulsive type). Patients were randomized to fixed-dose treatment groups receiving final doses of 10, 20, or 30 mg of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules or placebo once daily in the morning for three weeks. Significant improvements in patient behavior, based upon teacher ratings of attention and hyperactivity, were observed for all dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules doses compared to patients who received placebo, for all three weeks, including the first week of treatment, when all dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules subjects were receiving a dose of 10 mg/day. Patients who received dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules showed behavioral improvements in both morning and afternoon assessments compared to patients on placebo.
In a classroom analogue study, patients (N=51) receiving fixed doses of 10 mg, 20 mg, or 30 mg dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules demonstrated statistically significant improvements in teacher-rated behavior and performance measures, compared to patients treated with placebo.
A double-blind, randomized, multi-center, parallel-group, placebo-controlled study was conducted in adolescents aged 13 to 17 (N=327) who met DSM-IV® criteria for ADHD. The primary cohort of patients (n=287, weighing ≤ 75 kg/165 lbs) was randomized to fixed-dose treatment groups and received four weeks of treatment. Patients were randomized to receive final doses of 10 mg, 20 mg, 30 mg, and 40 mg dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules or placebo once daily in the morning. Patients randomized to doses greater than 10 mg were titrated to their final doses by 10 mg each week. The secondary cohort consisted of 40 subjects weighing >75 kg/165 lbs who were randomized to fixed-dose treatment groups receiving final doses of 50 mg and 60 mg dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules or placebo once daily in the morning for 4 weeks. The primary efficacy variable was the Attention Deficit Hyperactivity Disorder-Rating Scale IV (ADHD-RS-IV) total score for the primary cohort. The ADHD-RS-IV is an 18-item scale that measures the core symptoms of ADHD. Improvements in the primary cohort were statistically significantly greater in all four primary cohort active treatment groups (dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules 10 mg, 20 mg, 30 mg, and 40 mg) compared with the placebo group. There was not adequate evidence that doses greater than 20 mg/day conferred additional benefit.
Adult Patients
A double-blind, randomized, placebo-controlled, parallel-group study was conducted in adults (N=255) who met DSM-IV® criteria for ADHD. Patients were randomized to fixed-dose treatment groups receiving final doses of 20, 40, or 60 mg of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules or placebo once daily in the morning for four weeks. Significant improvements, measured with the Attention Deficit Hyperactivity Disorder-Rating Scale (ADHD-RS), an 18-item scale that measures the core symptoms of ADHD, were observed at endpoint for all dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules doses compared to patients who received placebo for all four weeks. There was not adequate evidence that doses greater than 20 mg/day conferred additional benefit.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
5 mg capsules: Blue Opaque Cap/Beige Opaque Body Hard Gelatin Capsule. Printed Laterally with "RP" on the cap and "705" on the body in black ink containing white to off-white beads. Bottles of 100 capsules NDC: 42858-055-01 10 mg capsules: Blue Opaque Cap/Blue Opaque Body Hard Gelatin Capsule. Printed Laterally with "RP" on the cap and "710" on the body in black ink containing white to off-white beads. Bottles of 100 capsules NDC: 42858-610-01 15 mg capsules: White Opaque Cap/Blue Opaque Body Hard Gelatin Capsule. Printed Laterally with "RP" on the cap and "715" on the body in black ink containing white to off-white beads. Bottles of 100 capsules NDC: 42858-215-01 20 mg capsules: Orange Opaque Cap/ Orange Opaque Body Hard Gelatin Capsule. Printed Laterally with "RP" on the cap and "720" on the body in black ink containing white to off-white beads. Bottles of 100 capsules NDC: 42858-720-01 25 mg capsules: White Opaque Cap/Orange Opaque Body Hard Gelatin Capsule. Printed Laterally with "RP" on the cap and "725" on the body in black ink containing white to off-white beads. Bottles of 100 capsules NDC: 42858-325-01 30 mg capsules: Orange Opaque Cap/Beige Opaque Body Hard Gelatin Capsule. Printed Laterally with "RP" on the cap and "730" on the body in black ink containing white to off-white beads. Bottles of 100 capsules NDC: 42858-930-01 Dispense in a tightly-closed, light-resistant container as defined in the USP, with a child-resistant closure, as required.
Store at 20ºC to 25°C (68°F to 77ºF) [see USP Controlled Room Temperature].
Disposal
Comply with local laws and regulations on drug disposal of CNS stimulants. Dispose of remaining, unused, or expired dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules at authorized collection sites such as retail pharmacies, hospital or clinic pharmacies, and law enforcement locations. If no take-back program or authorized collector is available, mix dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules with an undesirable, nontoxic substance to make it less appealing to children and pets. Place the mixture in a container such as a sealed plastic bag and discard dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules in the household trash.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Controlled Substance Status/Potential for Abuse, Misuse, and Dependence
Advise patients that dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules are a federally controlled substance because it can be abused or lead to dependence. Additionally, emphasize that dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules should be stored in a safe place to prevent misuse and/or abuse. Evaluate patient history (including family history) of abuse or dependence on alcohol, prescription medicines, or illicit drugs [see Warnings and Precautions (5.1), Drug Abuse and Dependence (9)].
Serious Cardiovascular Risks
Advise patients of serious cardiovascular risk (including sudden death, myocardial infarction, stroke, and hypertension) with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules. Patients who develop symptoms such as exertional chest pain, unexplained syncope, or other symptoms suggestive of cardiac disease during treatment should undergo a prompt cardiac evaluation [see Warnings and Precautions (5.1)].
Psychiatric Risks
Prior to initiating treatment with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, adequately screen patients with comorbid depressive symptoms to determine if they are at risk for bipolar disorder. Such screening should include a detailed psychiatric history, including a family history of suicide, bipolar disorder, and/or depression. Additionally, dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules therapy at usual doses may cause treatment-emergent psychotic or manic symptoms in patients without prior history of psychotic symptoms or mania [see Warnings and Precautions (5.2)].
Circulation Problems in Fingers and Toes [Peripheral Vasculopathy, including Raynaud's Phenomenon]
Instruct patients beginning treatment with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules about the risk of peripheral vasculopathy, including Raynaud's Phenomenon, and in associated signs and symptoms: fingers or toes may feel numb, cool, painful, and/or may change color from pale, to blue, to red. Instruct patients to report to their physician any new numbness, pain, skin color change, or sensitivity to temperature in fingers or toes. Instruct patients to call their physician immediately with any signs of unexplained wounds appearing on fingers or toes while taking dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules. Further clinical evaluation (e.g., rheumatology referral) may be appropriate for certain patients [see Warnings and Precautions (5.5)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome with concomitant use of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules and other serotonergic drugs including SSRIs, SNRIs, triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, St. John's Wort, and with drugs that impair metabolism of serotonin (in particular MAOIs, both those intended to treat psychiatric disorders and also others such as linezolid [see Contraindications (4), Warnings and Precautions (5.6) and Drug Interactions (7.1)]. Advise patients to contact their healthcare provider or report to the emergency room if they experience signs or symptoms of serotonin syndrome.
Concomitant Medications
Advise patients to notify their physicians if they are taking, or plan to take, any prescription or over-the-counter drugs because there is a potential for interactions [see Drug Interactions (7.1)].
Growth
Monitor growth in children during treatment with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, and patients who are not growing or gaining weight as expected may need to have their treatment interrupted [see Warnings and Precautions (5.3)].
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules during pregnancy [see Use in Specific Populations (8.1)].
Pregnancy
Advise patients to notify their healthcare providers if they become pregnant or intend to become pregnant during treatment with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules. Advise patients of the potential fetal effects from the use of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules during pregnancy [see Use in Specific Populations (8.1)].
Lactation
Advise women not to breastfeed if they are taking dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules [see Use in Specific Populations (8.2)].
Impairment in Ability to Operate Machinery or Vehicles
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules may impair the ability of the patient to engage in potentially hazardous activities such as operating machinery or vehicles; the patient should therefore be cautioned accordingly.
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
Dextroamphetamine Saccharate, Amphetamine Aspartate Monohydrate, Dextroamphetamine Sulfate, and Amphetamine Sulfate extended-release capsules, CII
(DEX-troe am FET-uh-meen Sack-uh-rate/ am-FET-uh-meen as-PAR-tate MAH-no-HIGH-drate/ DEX-troe-am-FET-uh-meen SULL-fate/am-FET-uh-meen-SULL-fate)Read the Medication Guide that comes with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules before you or your child starts taking it and each time you get a refill. There may be new information. This Medication Guide does not take the place of talking to your doctor about you or your child's treatment with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules.
What is the most important information I should know about dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules?
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules is a stimulant medicine. The following have been reported with use of stimulant medicines.
1. Heart-related problems:
- sudden death in patients who have heart problems or heart defects
- stroke and heart attack in adults
- increased blood pressure and heart rate
Tell your doctor if you or your child have any heart problems, heart defects, high blood pressure, or a family history of these problems.
Your doctor should check you or your child carefully for heart problems before starting dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules.
Your doctor should check you or your child's blood pressure and heart rate regularly during treatment with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules.
Call your doctor right away if you or your child has any signs of heart problems such as chest pain, shortness of breath, or fainting while taking dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules.
2. Mental (Psychiatric) problems:
All Patients
- new or worse behavior and thought problems
- new or worse bipolar illness
- new or worse aggressive behavior or hostility
Children and Teenagers
- new psychotic symptoms (such as hearing voices, believing things that are not true, are suspicious) or new manic symptoms
Tell your doctor about any mental problems you or your child have, or about a family history of suicide, bipolar illness, or depression.
Call your doctor right away if you or your child have any new or worsening mental symptoms or problems while taking dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules especially seeing or hearing things that are not real, believing things that are not real, or are suspicious.
3. Circulation problems in fingers and toes [Peripheral vasculopathy, including Raynaud's phenomenon]:
- Fingers or toes may feel numb, cool, painful
- Fingers or toes may change from pale, to blue, to red
Tell your doctor if you have or your child has numbness, pain, skin color change, or sensitivity to temperature in your fingers or toes.
Call your doctor right away if you have or your child has any unexplained wounds appearing on fingers or toes while taking dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules.
What is dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules?
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules is a once daily central nervous system stimulant prescription medicine. It is used for the treatment of Attention Deficit Hyperactivity Disorder (ADHD). Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules may help increase attention and decrease impulsiveness and hyperactivity in patients with ADHD.
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules should be used as a part of a total treatment program for ADHD that may include counseling or other therapies.
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules are a federally controlled substance (CII) because it can be abused or lead to dependence. Keep dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules in a safe place to prevent misuse and abuse. Selling or giving away dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules may harm others, and is against the law.
Tell your doctor if you or your child have (or have a family history of) ever abused or been dependent on alcohol, prescription medicines or street drugs.
Who should not take dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules?
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules should not be taken if you or your child:
- have heart disease or hardening of the arteries
- have moderate to severe high blood pressure
- have hyperthyroidism
- have an eye problem called glaucoma
- are very anxious, tense, or agitated
- have a history of drug abuse
- are taking or have taken within the past 14 days an anti-depression medicine called a monoamine oxidase inhibitor or MAOI.
- is sensitive to, allergic to, or had a reaction to other stimulant medicines
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules have not been studied in children less than 6 years old.
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules may not be right for you or your child. Before starting Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules tell you or your child's doctor about all health conditions (or a family history of) including if you or your child:
- have heart problems, heart defects, or high blood pressure
- have mental problems including psychosis, mania, bipolar illness, or depression
- have tics or Tourette's syndrome
- have liver problems
- have kidney problems
- have end stage renal disease (ESRD)
- have thyroid problems
- have seizures or have had an abnormal brain wave test (EEG)
- have circulation problems in fingers and toes
- are pregnant or plan to become pregnant. It is not known if dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules will harm your unborn baby.
- There is a pregnancy registry for females who are exposed to dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules during pregnancy. The purpose of the registry is to collect information about the health of females exposed to dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules and their baby. If you or your child becomes pregnant during treatment with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules, talk to your healthcare provider about registering with the National Pregnancy Registry of Psychostimulants at 1-866-961-2388 or visit online at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/othermedications/.
- are breastfeeding or plan to breastfeed. Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules passes into breast milk. You or your child should not breastfeed during treatment with dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules.
Can dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules be taken with other medicines?
Tell your doctor about all of the medicines that you or your child takes including prescription and non-prescription medicines, vitamins, and herbal supplements. Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules and some medicines may interact with each other and cause serious side effects. Sometimes the doses of other medicines will need to be adjusted while taking dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules
Your doctor will decide whether dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules can be taken with other medicines.
Especially tell your doctor if you or your child takes:
- anti-depression medicines including MAOIs
- anti-psychotic medicines
- lithium
- narcotic pain medicines
- seizure medicines
- blood thinner medicines
- blood pressure medicines
- stomach acid medicines
- cold or allergy medicines that contain decongestants
Know the medicines that you or your child takes. Keep a list of your medicines with you to show your doctor and pharmacist.
Do not start any new medicine while taking dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules without talking to your doctor first.
How should dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules be taken?
- Take dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules exactly as prescribed. Your doctor may adjust the dose until it is right for you or your child.
- Take dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules once a day in the morning when you first wake up. Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules is an extended release capsule. It releases medicine into your body throughout the day.
- Swallow dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules whole with water or other liquids. If you or your child cannot swallow the capsule, open it and sprinkle the medicine over a spoonful of applesauce. Swallow all of the applesauce and medicine mixture without chewing immediately. Follow with a drink of water or other liquid. Never chew or crush the capsule or the medicine inside the capsule.
- Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules can be taken with or without food.
- From time to time, your doctor may stop dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules treatment for a while to check ADHD symptoms.
- Your doctor may do regular checks of the blood, heart, and blood pressure while taking dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules. Children should have their height and weight checked often while taking dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules. Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules treatment may be stopped if a problem is found during these check-ups.
- If you or your child takes too much dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules or overdoses, call your doctor or poison control center right away, or get emergency treatment.
What are possible side effects of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules?
See "What is the most important information I should know about dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules?" for information on reported heart and mental problems.
Other serious side effects include:
- slowing of growth (height and weight) in children
- seizures, mainly in patients with a history of seizures
- eyesight changes or blurred vision
Common side effects include:
- headache
- decreased appetite
- stomach ache
- nervousness
- trouble sleeping
- mood swings
- weight loss
- dizziness
- dry mouth
- fast heart beat
Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules may affect you or your child's ability to drive or do other dangerous activities.
Talk to your doctor if you or your child has side effects that are bothersome or do not go away.
This is not a complete list of possible side effects. Ask your doctor or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules?
- Store dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules in a safe place at room temperature, 68°F to 77ºF (20ºC to 25°C) [see USP Controlled Room Temperature].
- Keep dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules and all medicines out of the reach of children.
General information about dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules for a condition for which it was not prescribed. Do not give dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules to other people, even if they have the same condition. It may harm them and it is against the law.
This Medication Guide summarizes the most important information about dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules that was written for healthcare professionals. For more information, you may also contact Rhodes Pharmaceuticals L.P. (the maker of dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules) at 1-888-827-0616 or visit the website at http://www.rhodespharma.com
What are the ingredients in dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules?
Active Ingredients: Dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, USP, and amphetamine sulfate USP
Inactive Ingredients: The inactive ingredients in dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate extended-release capsules include: sugar spheres (sucrose, sugar syrup and corn starch), hypromellose, talc, triethyl citrate, methacrylic acid copolymer dispersion and gelatin capsules. Gelatin capsules contain: titanium dioxide, and FD&C Blue# 1. The 5 mg, 20 mg, 25 mg, and 30 mg capsules also contain FD&C Yellow#6. The 5 mg, 10 mg, and 15 mg also contain D&C Yellow#10 and D&C Red#33. Additionally, the imprint ink contains: shellac, black iron oxide, and potassium hydroxide.
Marketed by:
Rhodes Pharmaceuticals L.P., Coventry, RI 02816Rev: 07/2019
This Medication Guide has been approved by the U.S. Food and Drug Administration.
-
PRINCIPAL DISPLAY PANEL - 5 mg Capsule Bottle Label
NDC: 42858-055-01
CII
Dextroamphetamine
Saccharate, Amphetamine
Aspartate Monohydrate,
Dextroamphetamine Sulfate,
and Amphetamine Sulfate
Extended-Release Capsules(Mixed Salts of a Single-Entity
Amphetamine Product)5 mg*
Rx only
PHARMACIST: Dispense the Accompanying
Medication Guide to Each Patient.Marketed by:
Rhodes Pharmaceuticals L.P., Coventry, RI 02816100 Capsules
-
PRINCIPAL DISPLAY PANEL - 10 mg Capsule Bottle Label
NDC: 42858-610-01
CII
Dextroamphetamine
Saccharate, Amphetamine
Aspartate Monohydrate,
Dextroamphetamine Sulfate,
and Amphetamine Sulfate
Extended-Release Capsules(Mixed Salts of a Single-Entity
Amphetamine Product)10 mg*
Rx only
PHARMACIST: Dispense the Accompanying
Medication Guide to Each Patient.Marketed by:
Rhodes Pharmaceuticals L.P., Coventry, RI 02816100 Capsules
-
PRINCIPAL DISPLAY PANEL - 15 mg Capsule Bottle Label
NDC: 42858-215-01
CII
Dextroamphetamine
Saccharate, Amphetamine
Aspartate Monohydrate,
Dextroamphetamine Sulfate,
and Amphetamine Sulfate
Extended-Release Capsules(Mixed Salts of a Single-Entity
Amphetamine Product)15 mg*
Rx only
PHARMACIST: Dispense the Accompanying
Medication Guide to Each Patient.Marketed by:
Rhodes Pharmaceuticals L.P., Coventry, RI 02816100 Capsules
-
PRINCIPAL DISPLAY PANEL - 20 mg Capsule Bottle Label
NDC: 42858-720-01
CII
Dextroamphetamine
Saccharate, Amphetamine
Aspartate Monohydrate,
Dextroamphetamine Sulfate,
and Amphetamine Sulfate
Extended-Release Capsules(Mixed Salts of a Single-Entity
Amphetamine Product)20 mg*
Rx only
PHARMACIST: Dispense the Accompanying
Medication Guide to Each Patient.Marketed by:
Rhodes Pharmaceuticals L.P., Coventry, RI 02816100 Capsules
-
PRINCIPAL DISPLAY PANEL - 25 mg Capsule Bottle Label
NDC: 42858-325-01
CII
Dextroamphetamine
Saccharate, Amphetamine
Aspartate Monohydrate,
Dextroamphetamine Sulfate,
and Amphetamine Sulfate
Extended-Release Capsules(Mixed Salts of a Single-Entity
Amphetamine Product)25 mg*
Rx only
PHARMACIST: Dispense the Accompanying
Medication Guide to Each Patient.Marketed by:
Rhodes Pharmaceuticals L.P., Coventry, RI 02816100 Capsules

-
PRINCIPAL DISPLAY PANEL - 30 mg Capsule Bottle Label
NDC: 42858-930-01
CII
Dextroamphetamine
Saccharate, Amphetamine
Aspartate Monohydrate,
Dextroamphetamine Sulfate,
and Amphetamine Sulfate
Extended-Release Capsules(Mixed Salts of a Single-Entity
Amphetamine Product)30 mg*
Rx only
PHARMACIST: Dispense the Accompanying
Medication Guide to Each Patient.Marketed by:
Rhodes Pharmaceuticals L.P., Coventry, RI 02816100 Capsules
-
INGREDIENTS AND APPEARANCE
DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE, AND AMPHETAMINE SULFATE EXTENDED-RELEASE
dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42858-055 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROAMPHETAMINE SACCHARATE (UNII: G83415V073) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SACCHARATE 1.25 mg AMPHETAMINE ASPARTATE MONOHYDRATE (UNII: O1ZPV620O4) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE ASPARTATE MONOHYDRATE 1.25 mg DEXTROAMPHETAMINE SULFATE (UNII: JJ768O327N) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SULFATE 1.25 mg AMPHETAMINE SULFATE (UNII: 6DPV8NK46S) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE SULFATE 1.25 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) Hypromellose, UNSPECIFIED (UNII: 3NXW29V3WO) Talc (UNII: 7SEV7J4R1U) Triethyl Citrate (UNII: 8Z96QXD6UM) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) WATER (UNII: 059QF0KO0R) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Shellac (UNII: 46N107B71O) Isopropyl alcohol (UNII: ND2M416302) Tert-butyl alcohol (UNII: MD83SFE959) Propylene glycol (UNII: 6DC9Q167V3) Ammonia (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Potassium hydroxide (UNII: WZH3C48M4T) Product Characteristics Color BLUE (Opaque) , BROWN (Beige Opaque) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code RP;705 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42858-055-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/22/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210651 08/22/2019 DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE, AND AMPHETAMINE SULFATE EXTENDED-RELEASE
dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42858-610 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROAMPHETAMINE SACCHARATE (UNII: G83415V073) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SACCHARATE 2.5 mg AMPHETAMINE ASPARTATE MONOHYDRATE (UNII: O1ZPV620O4) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE ASPARTATE MONOHYDRATE 2.5 mg DEXTROAMPHETAMINE SULFATE (UNII: JJ768O327N) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SULFATE 2.5 mg AMPHETAMINE SULFATE (UNII: 6DPV8NK46S) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE SULFATE 2.5 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) Hypromellose, UNSPECIFIED (UNII: 3NXW29V3WO) Talc (UNII: 7SEV7J4R1U) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) WATER (UNII: 059QF0KO0R) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Shellac (UNII: 46N107B71O) Isopropyl alcohol (UNII: ND2M416302) Tert-butyl alcohol (UNII: MD83SFE959) Propylene glycol (UNII: 6DC9Q167V3) Ammonia (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Potassium hydroxide (UNII: WZH3C48M4T) Product Characteristics Color BLUE (Opaque) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code RP;710 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42858-610-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/22/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210651 08/22/2019 DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE, AND AMPHETAMINE SULFATE EXTENDED-RELEASE
dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42858-215 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROAMPHETAMINE SACCHARATE (UNII: G83415V073) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SACCHARATE 3.75 mg AMPHETAMINE ASPARTATE MONOHYDRATE (UNII: O1ZPV620O4) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE ASPARTATE MONOHYDRATE 3.75 mg DEXTROAMPHETAMINE SULFATE (UNII: JJ768O327N) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SULFATE 3.75 mg AMPHETAMINE SULFATE (UNII: 6DPV8NK46S) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE SULFATE 3.75 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) Hypromellose, UNSPECIFIED (UNII: 3NXW29V3WO) Talc (UNII: 7SEV7J4R1U) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) WATER (UNII: 059QF0KO0R) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Shellac (UNII: 46N107B71O) Isopropyl alcohol (UNII: ND2M416302) Tert-butyl alcohol (UNII: MD83SFE959) Propylene glycol (UNII: 6DC9Q167V3) Ammonia (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Potassium hydroxide (UNII: WZH3C48M4T) Product Characteristics Color WHITE (Opaque) , BLUE (Opaque) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code RP;715 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42858-215-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/22/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210651 08/22/2019 DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE, AND AMPHETAMINE SULFATE EXTENDED-RELEASE
dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42858-720 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROAMPHETAMINE SACCHARATE (UNII: G83415V073) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SACCHARATE 5 mg AMPHETAMINE ASPARTATE MONOHYDRATE (UNII: O1ZPV620O4) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE ASPARTATE MONOHYDRATE 5 mg DEXTROAMPHETAMINE SULFATE (UNII: JJ768O327N) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SULFATE 5 mg AMPHETAMINE SULFATE (UNII: 6DPV8NK46S) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE SULFATE 5 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) Hypromellose, UNSPECIFIED (UNII: 3NXW29V3WO) Talc (UNII: 7SEV7J4R1U) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) WATER (UNII: 059QF0KO0R) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Shellac (UNII: 46N107B71O) Isopropyl alcohol (UNII: ND2M416302) Tert-butyl alcohol (UNII: MD83SFE959) Propylene glycol (UNII: 6DC9Q167V3) Ammonia (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Potassium hydroxide (UNII: WZH3C48M4T) Product Characteristics Color ORANGE (Opaque) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code RP;720 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42858-720-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/22/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210651 08/22/2019 DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE, AND AMPHETAMINE SULFATE EXTENDED-RELEASE
dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42858-325 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROAMPHETAMINE SACCHARATE (UNII: G83415V073) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SACCHARATE 6.25 mg AMPHETAMINE ASPARTATE MONOHYDRATE (UNII: O1ZPV620O4) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE ASPARTATE MONOHYDRATE 6.25 mg DEXTROAMPHETAMINE SULFATE (UNII: JJ768O327N) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SULFATE 6.25 mg AMPHETAMINE SULFATE (UNII: 6DPV8NK46S) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE SULFATE 6.25 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) Hypromellose, UNSPECIFIED (UNII: 3NXW29V3WO) Talc (UNII: 7SEV7J4R1U) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) WATER (UNII: 059QF0KO0R) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Shellac (UNII: 46N107B71O) Isopropyl alcohol (UNII: ND2M416302) Tert-butyl alcohol (UNII: MD83SFE959) Propylene glycol (UNII: 6DC9Q167V3) Ammonia (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Potassium hydroxide (UNII: WZH3C48M4T) Product Characteristics Color WHITE (Opaque) , ORANGE (Opaque) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code RP;725 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42858-325-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/22/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210651 08/22/2019 DEXTROAMPHETAMINE SACCHARATE, AMPHETAMINE ASPARTATE MONOHYDRATE, DEXTROAMPHETAMINE SULFATE, AND AMPHETAMINE SULFATE EXTENDED-RELEASE
dextroamphetamine saccharate, amphetamine aspartate monohydrate, dextroamphetamine sulfate, and amphetamine sulfate capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42858-930 Route of Administration ORAL DEA Schedule CII Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROAMPHETAMINE SACCHARATE (UNII: G83415V073) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SACCHARATE 7.5 mg AMPHETAMINE ASPARTATE MONOHYDRATE (UNII: O1ZPV620O4) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE ASPARTATE MONOHYDRATE 7.5 mg DEXTROAMPHETAMINE SULFATE (UNII: JJ768O327N) (DEXTROAMPHETAMINE - UNII:TZ47U051FI) DEXTROAMPHETAMINE SULFATE 7.5 mg AMPHETAMINE SULFATE (UNII: 6DPV8NK46S) (AMPHETAMINE - UNII:CK833KGX7E) AMPHETAMINE SULFATE 7.5 mg Inactive Ingredients Ingredient Name Strength SUCROSE (UNII: C151H8M554) Hypromellose, UNSPECIFIED (UNII: 3NXW29V3WO) Talc (UNII: 7SEV7J4R1U) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) WATER (UNII: 059QF0KO0R) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Shellac (UNII: 46N107B71O) Isopropyl alcohol (UNII: ND2M416302) Tert-butyl alcohol (UNII: MD83SFE959) Propylene glycol (UNII: 6DC9Q167V3) Ammonia (UNII: 5138Q19F1X) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Potassium hydroxide (UNII: WZH3C48M4T) Product Characteristics Color ORANGE (Opaque) , BROWN (Beige Opaque) Score no score Shape CAPSULE Size 22mm Flavor Imprint Code RP;730 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42858-930-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/22/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA210651 08/22/2019 Labeler - Rhodes Pharmaceuticals L.P. (831928986)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.