NASCOBAL- cyanocobalamin spray
Nascobal by
Drug Labeling and Warnings
Nascobal by is a Prescription medication manufactured, distributed, or labeled by Par Pharmaceutical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NASCOBAL® NASAL SPRAY safely and effectively. See full prescribing information for NASCOBAL® NASAL SPRAY.
NASCOBAL (cyanocobalamin nasal spray)
Initial U.S. Approval: 1942
INDICATIONS AND USAGE
NASCOBAL is a vitamin B12 indicated for:
- Vitamin B12 maintenance therapy in adult patients with pernicious anemia who are in remission following intramuscular vitamin B12 therapy and who have no nervous system involvement(1)
- Treatment of adult patients with dietary, drug-induced, or malabsorption-related vitamin B12 deficiency not due to pernicious anemia (1)
- Prevention of vitamin B12 deficiency in adult patients with vitamin B12 requirements in excess of normal (1)
Limitations of Use:
- Should not be used for the vitamin B12 absorption test (Schilling test). (1)
- In patients with correctible or temporary causes of vitamin B12 deficiency the benefit of continued long-term use following correction of vitamin B12 deficiency and underlying disease has not been established. (1)
- In patients with active symptoms of nasal congestion, allergic rhinitis or upper respiratory infection effectiveness has not been established. (1)
DOSAGE AND ADMINISTRATION
- Prior to treatment, obtain hematocrit, reticulocyte count, vitamin B12, folate, and iron levels. (2.1)
- The recommended initial dose is one spray (500 mcg) in one nostril once weekly.(2.2)
- Administer at least one hour before or one hour after ingestion of hot foods or liquids. (2.2)
- Monitor serum B12 levels periodically.Obtain a serum B12 level and peripheral blood count one month after treatment initiation, then subsequently at intervals of 3 to 6 months. (2.3)
- If serum levels of B12 decline after one month of treatment, consider increasing the dose. Assess serum B12 level one month after each dose adjustment.If serum B12 levels are persistently low, consider alternative therapy (e.g., intramuscular or subcutaneous vitamin B12 therapy). (2.3)
- See Full Prescibing Information to see what other therapies should be administered with NASCOBAL. (2.4)
DOSAGE FORMS AND STRENGTHS
Nasal spray: 500 mcg cyanocobalamin/0.1 mL (per actuation) (3)
CONTRAINDICATIONS
Hypersensitivity to cobalt, vitamin B12 or any excipients (4)
WARNINGS AND PRECAUTIONS
Severe Optic Atrophy in Patients with Leber’s Disease:Patients with early Leber’s disease who were treated with vitamin B12 suffered severe and swift optic atrophy. NASCOBAL is not recommended for use in these patients.(5.1) (5)
Anaphylactic Reactions: Anaphylactic shock and death have been reported after parenteral vitamin B12 administration. If patients are to start NASCOBAL before having tolerated cyanocobalamin parenterally, consider administering an intradermal test dose of parenteral vitamin B12 to patients suspected of cyanocobalamin hypersensitivity. (2.1,5.2) (5)
Masking of Folate Deficiency with Vitamin B12 Use: Doses of vitamin B12 exceeding 10 mcg daily may produce hematologic response in patients with folate deficient megaloblastic anemia and may therefore mask a previously unrecognized folate deficiency. Assess both vitamin B12 and folate levels prior to initiating therapy with NASCOBAL or with folic acid. (5.3) (5)
Hypokalemia and Thrombocytosis Due to Intense Treatment of Megaloblastic Anemia: Hypokalemia and sudden death may occur in severe megaloblastic anemia that is treated intensely with vitamin B12. Monitor serum potassium levels and platelet count during therapy. (5.4) (5)
Unmasking of Polycythemia Vera:Vitamin B12 deficiency may suppress the signs of polycythemia vera. Treatment with NASCOBAL may unmask this condition. Patients exhibiting clinical or hematologic response consistent with polycythemia vera should be referred for further evaluation. (5.5) (5)
ADVERSE REACTIONS
The most common adverse reactions (≥ 4%) were infection, headache, glossitis, paresthesia, asthenia, nausea and rhinitis (6.1) (6)
To report SUSPECTED ADVERSE REACTIONS, contact Par Pharmaceutical, Inc. at 1-800-828-9393 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling (6)
USE IN SPECIFIC POPULATIONS
See Patient Counseling Information. (8)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Testing and Other Considerations Prior to Dosing
2.2 Recommended Dosage
2.3 Monitoring, Dosage Modifications, and Treatment Duration
2.4 Administration of NASCOBAL with Other Therapy
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Severe Optic Atrophy in Patients with Leber’s Disease
5.2 Anaphylactic Reactions
5.3 Masking of Folate Deficiency with Vitamin B12 Use
5.4 Hypokalemia and Thrombocytosis Due to Intense Treatment of Megaloblastic Anemia
5.5 Unmasking of Polycythemia Vera
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis and Mutagenesis and Impairment of Fertility
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
NASCOBAL is indicated for:
- Vitamin B12 maintenance therapy in adult patients with pernicious anemia who are in remission following intramuscular vitamin B12 therapy and who have no nervous system involvement
- Treatment of adult patients with dietary, drug-induced, or malabsorption-related vitamin B12 deficiency not due to pernicious anemia
- Prevention of vitamin B12 deficiency in adult patients with vitamin B12 requirements in excess of normal
Limitations of Use
- NASCOBAL should not be used for the vitamin B12 absorption test (Schilling test).
- In patients with correctible or temporary causes of vitamin B12 deficiency, the benefit of continued long-term use of NASCOBAL following adequate correction of vitamin B12 deficiency and underlying disease has not been established.
- The effectiveness of NASCOBAL in patients with active symptoms of nasal congestion, allergic rhinitis or upper respiratory infection has not been determined. Treatment with NASCOBAL should be deferred until symptoms have subsided.
-
2 DOSAGE AND ADMINISTRATION
2.1 Testing and Other Considerations Prior to Dosing
Prior to treatment, obtain hematocrit, reticulocyte count, vitamin B12, folate, and iron levels [see Dosage and Administration (2.4)]. Consider the potential for concomitant drugs to interfere with vitamin B12 and folate diagnostic blood assays [see Drug Interactions (7)].
In patients with suspected cobalamin hypersensitivity, consider administering an intradermal test dose of parenteral vitamin B12 prior to use of NASCOBAL [see Warnings and Precautions (5.2)].
2.2 Recommended Dosage
The recommended initial dose of NASCOBAL is one spray (500 mcg) administered in ONE nostril once weekly. Administer NASCOBAL at least one hour before or one hour after ingestion of hot foods or liquids since hot foods may cause nasal secretions and a resulting loss of medication. Defer use of NASCOBAL in patients with nasal congestion, allergic rhinitis, or upper respiratory infections until after symptoms have subsided.
2.3 Monitoring, Dosage Modifications, and Treatment Duration
Monitoring for Response and Safety
Monitor serum B12 levels periodically during therapy to establish adequacy of therapy. Obtain a serum B12 level and peripheral blood count one month after treatment initiation, then subsequently at intervals of 3 to 6 months [see Warnings and Precautions (5.3)].
Dosage Modifications
If serum levels of B12 decline after one month of treatment with NASCOBAL, consider increasing the dose. Assess serum B12 level one month after each dose adjustment. If serum B12 levels are persistently low, consider alternative therapy (e.g., intramuscular or subcutaneous vitamin B12 therapy).
Treatment Duration
In patients whose underlying cause of vitamin B12 deficiency has been corrected and are deemed no longer at risk for vitamin B12 deficiency, discontinue NASCOBAL. The safety and effectiveness of continued long-term use in these individuals has not been established.
In patients with pernicious anemia, continue appropriate vitamin B12 treatment indefinitely.
2.4 Administration of NASCOBAL with Other Therapy
NASCOBAL should be administered with other therapy(ies) in:
- Patients with concurrent folate and vitamin B12 deficiency:Administer folic acid in addition to NASCOBAL
- Patients with concurrent iron and vitamin B12 deficiency:Administer iron in addition to NASCOBAL
- Patients with correctible causes of vitamin B12 deficiency:Consider measures to treat the underlying condition associated with vitamin B12 deficiency in addition to treatment with NASCOBAL
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
NASCOBAL is contraindicated in patients with hypersensitivity to cobalt and/or vitamin B12 or any of its excipients [see Warnings and Precautions (5.2)]. Anaphylactic shock and death have been reported after parenteral vitamin B12 administration in sensitive patients.
-
5 WARNINGS AND PRECAUTIONS
5.1 Severe Optic Atrophy in Patients with Leber’s Disease
Patients with early Leber’s disease (hereditary optic nerve atrophy) who were treated with vitamin B12 suffered severe and swift optic atrophy. Cyanocobalamin products, including NASCOBAL, is not recommended for use in patients with Leber’s optic atrophy. For patients with Leber’s disease requiring vitamin B12, consider alternative therapy (e.g., hydroxocobalamin) for B12 supplementation.
5.2 Anaphylactic Reactions
Anaphylactic shock and death have been reported after parenteral vitamin B12 administration. If patients are to start NASCOBAL before having tolerated cyanocobalamin parenterally, consider administering an intradermal test dose of parenteral vitamin B12 to patients suspected of cyanocobalamin hypersensitivity [see Dosage and Administration (2.1)].
5.3 Masking of Folate Deficiency with Vitamin B12 Use
Doses of vitamin B12 exceeding 10 mcg daily may produce hematologic response in patients with folate deficient megaloblastic anemia and may therefore mask a previously unrecognized folate deficiency. Vitamin B12 is not a substitute for folic acid [see Dosage and Administration (2.4)]. Assess both vitamin B12 and folate levels prior to initiating therapy with vitamin B12, including NASCOBAL, or with folic acid [see Dosage and Administration (2.1)].
5.4 Hypokalemia and Thrombocytosis Due to Intense Treatment of Megaloblastic Anemia
Hypokalemia and sudden death may occur in severe megaloblastic anemia that is treated intensely with vitamin B12. Hypokalemia and thrombocytosis can occur upon conversion of severe megaloblastic anemia to normal erythropoiesis with vitamin B12 therapy. Therefore, serum potassium levels and platelet count should be monitored carefully during therapy [see Dosage and Administration (2.3)].
-
6 ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in the labeling:
- Severe Optic Atrophy in Patients with Leber’s Disease [see Warnings and Precautions (5.1)].
- Anaphylactic Reactions [see Warnings and Precautions (5.2)].
- Hypokalemia and Thrombocytosis Due to Intense Treatment of Megaloblastic Anemia [see Warnings and Precautions (5.4)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reactions described in Table 1 below are based on data from an eight week cross over trial in which vitamin B12 deficient patients in hematologic remission received one vitamin B12 intramuscular injection (N=25) and then received once weekly intranasal administration of another nasal cyanocobalamin formulation (N=24) for 4 weeks.
Table 1. Adverse Reactions Following Intranasal or Intramuscular Administration of Cyanocobalamin In Vitamin B12 Deficient Patients in Hematologic Remission Adverse Reaction Number of Patients (%) Another Cyanocobalamin Nasal Formulation, 500 mcg
(n=24)Intramuscular Cyanocobalamin*, 100 mcg
(n=25)Infection a 3 (13) 3 (12) Headache 1 (4) 5 (20) Asthenia 1 (4) 4 (16) Nausea 1 (4) 1 (4) Glossitis 1 (4) 0 (0) Paresthesia 1 (4) 1 (4) Rhinitis 1 (4) 2 (8) a Sore throat, common cold
* The data are not an adequate basis for comparison of rates between the study drug and the active control
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
The limited available data on NASCOBAL in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. However, vitamin B12 is an essential vitamin and requirements are increased during pregnancy.
Animal reproduction studies have not been conducted with vitamin B12.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
Vitamin B12 is present in the milk of lactating women in concentrations which approximate the mother’s vitamin B12 blood level. Vitamin B12 does not appear to pose more than a minimal risk to breastfeeding children.
8.5 Geriatric Use
Clinical studies of NASCOBAL did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
-
11 DESCRIPTION
Cyanocobalamin is a synthetic form of vitamin B12. The chemical name is 5,6-dimethyl-benzimidazolyl cyanocobamide. The cobalt content is 4.35%. The molecular formula is C63H88CoN14O14P, which corresponds to a molecular weight of 1355.38 and the following structural formula:
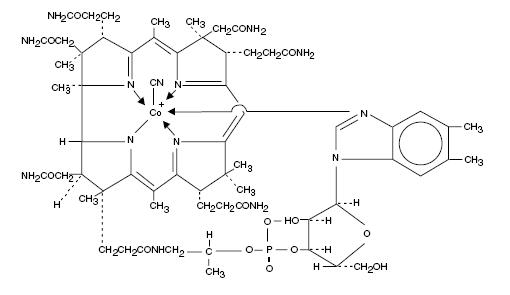
Figure 1. Nascobal Chemical Structure
Cyanocobalamin occurs as dark red crystals or orthorhombic needles or crystalline red powder. It is very hygroscopic in the anhydrous form, and sparingly to moderately soluble in water (1:80). Its pharmacologic activity is destroyed by heavy metals (iron) and strong oxidizing or reducing agents (vitamin C), but not by autoclaving for short periods of time (15-20 minutes) at 121°C. The vitamin B12 coenzymes are very unstable in light.
NASCOBAL (cyanocobalamin) nasal spray is a solution of cyanocobalamin, USP (vitamin B12) for administration as a spray to the nasal mucosa. Each single-use device of NASCOBAL NASAL SPRAY contains 0.125 mL of a 500 mcg/0.1 mL solution of cyanocobalamin with, benzalkonium chloride in purified water, citric acid, glycerin and sodium citrate. The spray solution has a pH between 4.5 and 5.5. Each spray delivers an average of 500 mcg of cyanocobalamin per actuation.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Vitamin B12 can be converted to coenzyme B12 in tissues, and as such is essential for conversion of methylmalonate to succinate and synthesis of methionine from homocysteine, a reaction which also requires folate. In the absence of coenzyme B12, tetrahydrofolate cannot be regenerated from its inactive storage form, 5-methyltetrahydrofolate, and a functional folate deficiency occurs. Vitamin B12 also may be involved in maintaining sulfhydryl (SH) groups in the reduced form required by many SH-activated enzyme systems. Through these reactions, vitamin B12 is associated with fat and carbohydrate metabolism and protein synthesis.
12.2 Pharmacodynamics
Parenteral (intramuscular) administration of vitamin B12 completely reverses the megaloblastic anemia and GI symptoms of vitamin B12 deficiency; the degree of improvement in neurologic symptoms depends on the duration and severity of the lesions, although progression of the lesions is immediately arrested. In pernicious anemia patients, once weekly intranasal dosing with 500 mcg B12 gel resulted in a consistent increase in pre-dose serum B12 levels during one month of treatment (p < 0.003) above that seen one month after 100 mcg intramuscular dose (Figure 2).
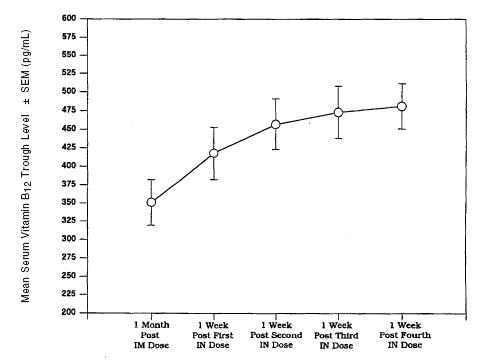
Figure2. Vitamin B12 Serum Trough Levels After Intramuscular Solution (IM) of 100 mcg and Nasal Gel (IN) Administration of 500 mcg Cyanocobalamin After Weekly Doses.
12.3 Pharmacokinetics
Absorption
Vitamin B12 is bound to intrinsic factor during transit through the stomach; separation occurs in the terminal ileum in the presence of calcium, and vitamin B12 enters the mucosal cell for absorption. It is then transported by the transcobalamin binding proteins. A small amount (approximately 1% of the total amount ingested) is absorbed by simple diffusion, but this mechanism is adequate only with very large doses.
A three way crossover study in 25 fasting healthy subjects was conducted to compare the bioavailability of the B12 nasal spray to the B12 nasal gel and to evaluate the relative bioavailability of the nasal formulations as compared to the intramuscular injection. The peak concentrations after administration of intranasal spray were reached in 1.25 +/- 1.9 hours. The mean peak plasma concentration (Cmax) of B12, obtained after baseline correction, following administration of intranasal spray were 748 +/-549 pg/mL. The bioavailability of the B12 nasal spray was found to be 10% less than the B12 nasal gel.
Distribution
In the blood, B12 is bound to transcobalamin II, a specific B-globulin carrier protein, and is distributed and stored primarily in the liver and bone marrow.
Elimination
About 3-8 mcg of B12 is secreted into the GI tract daily via the bile and undergoes some enterohepatic recycling; in normal subjects with sufficient intrinsic factor, all but about 1 mcg is reabsorbed. When B12 is administered in doses which saturate the binding capacity of plasma proteins and the liver, the unbound B12 is rapidly eliminated in the urine. Retention of B12 in the body is dose-dependent. About 80-90% of an intramuscular dose up to 50 mcg is retained in the body; this percentage drops to 55% for a 100 mcg dose, and decreases to 15% when a 1000 mcg dose is given.
- 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
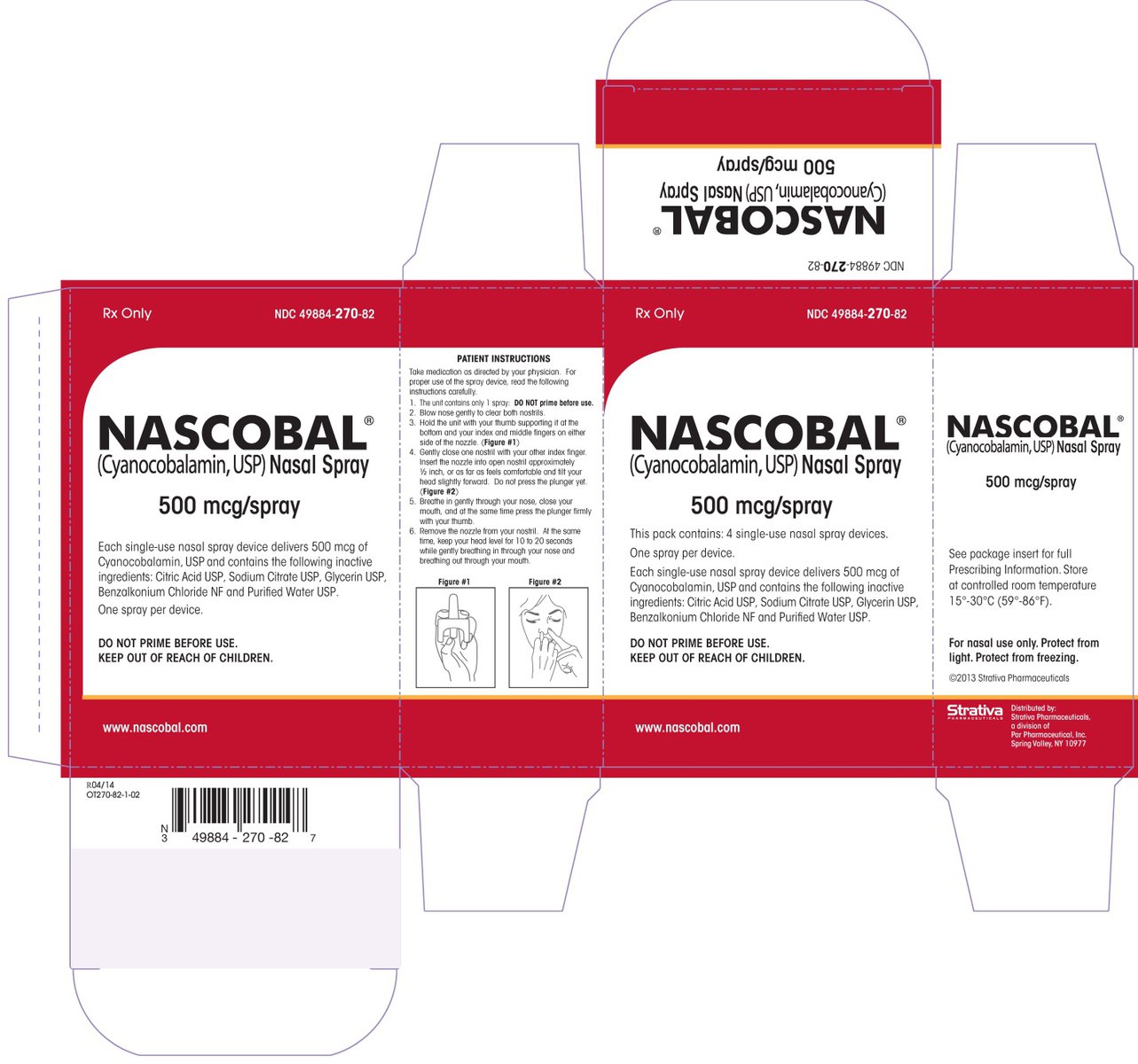
NASCOBAL is a nasal spray available in a dosage strength of 500 mcg cyanocobalamin/0.1 mL (per actuation). It is supplied in boxes of 4 single-use nasal spray devices and a package insert (NDC: 49884-270-82).
Protect from light. Keep covered in carton until ready to use. Store upright at controlled room temperature 15°C to 30°C (59°F to 86°F). Protect from freezing.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Importance of Adherence to Therapy and Follow-up
Advise patients of the importance of adhering to therapy to prevent the development, recurrence, or progression of anemia and the development or progression of neurological manifestations.
Patients experiencing symptomatic nasal congestion, allergic rhinitis or an upper respiratory infection should be advised to defer treatment with NASCOBAL until symptoms have subsided.
Counsel patients on the importance of returning for follow-up blood tests every 3 to 6 months to confirm adequacy of therapy [see Dosage and Administration (2.3)].
Specific Instructions for Administration
Advise patients to administer NASCOBAL at least one hour before or one hour after ingestion of hot foods or liquids since hot foods may cause nasal secretions and a resulting loss of medication.
Use of Nasal Spray Device
Provide patients with instructions on nasal administration of NASCOBAL. In addition to alerting patients to the instructions contained in the Instructions For Use and instructions supplied on each carton, demonstrate procedures for use to each patient.
-
PATIENT INFORMATION
NASCOBAL (NAZ. co. bal)
(cyanocobalamin) nasal spray
NASCOBAL is for use in your nose only.
What is NASCOBAL?
NASCOBAL is a prescription medicine used:
- for maintenance therapy to treat vitamin B12 deficiency (low levels of vitamin B12) in adults with pernicious anemia who achieved healthy vitamin B12 levels after receiving vitamin B12 shots and do not have nervous system problems.
- to treat vitamin B12 deficiency caused by:
- certain food limitations,
- oother medicines that cause vitamin B12 deficiency, or
- lack of absorption (malabsorption) that are not related to pernicious anemia.
- to prevent vitamin B12 deficiency in adults who require higher amounts of vitamin B12.
NASCOBAL should not be used for a vitamin B12 absorption test known as the Schilling test.
In people that have corrected or have fixed their short-term causes of vitamin B12 deficiency, the benefit of continued long-term use of NASCOBAL is not known after vitamin B12 deficiency has been corrected.
It is not known if NASCOBAL is effective in people that currently have a stuffy nose, allergies or an upper respiratory infection. You should wait until these symptoms have gone away before using NASCOBAL.
It is not known if NASCOBAL is safe and effective in children.
Do not use NASCOBAL if you are allergic to cobalt, vitamin B12, cyanocobalamin or any ingredients in NASCOBAL. See the end of this leaflet for a complete list of ingredients in NASCOBAL.
Before you use NASCOBAL, tell your healthcare provider about all of your medical conditions, including if you:
- have a history of a certain disease that effects or weakens the optic nerve in your eye (early Leber’s disease). NASCOBAL is not recommended in people with Leber’s optic atrophy.
- have had an allergic reaction to vitamin B12 injections or intravenous (IV) infusion.
- have low levels of folate or iron.
- are pregnant or plan to become pregnant. It is not known if NASCOBAL will harm your unborn baby.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use NASCOBAL?
- See the Instructions for Use at the end of this leaflet for instructions on how to use NASCOBAL.
- You should use NASCOBAL exactly as your healthcare provider tells you to use it.
- Your healthcare provider will tell you how much NASCOBAL to use and when to use it.
- You should not use NASCOBAL 1 hour before or 1 hour after eating hot foods or drinking hot liquids. Eating hot food or drinking hot liquids too soon before or after taking NASCOBAL can cause you to have a runny nose and can affect the amount of NASCOBAL you receive.
- Your healthcare provider may do certain blood tests before you use NASCOBAL and every 3 to 6 months while you are taking it.
- Your healthcare provider may change your dose based on the results of your blood tests.
What are the possible side effects of NASCOBAL?
NASCOBAL can cause serious side effects, including:
- Weaken your eye nerve in patients with early Leber’s disease. Tell your healthcare provider if you or your family has a history of early Leber’s disease.
- Serious allergic reactions. Serious life-threatening allergic reactions including death have happened in people who have received vitamin B12 by injection or by intravenous (IV) infusion (parenteral). Tell your healthcare provider if you have ever had a reaction after you received vitamin B12 by injection or IV infusion.
- Hiding (masking) a folate deficiency. Your healthcare provider may do certain blood tests to check your vitamin B12 and folate levels.
- Low potassium levels (hypokalemia) and low blood platelets (thrombocytopenia) can happen in people with serious megaloblastic anemia. Your healthcare provider may monitor your potassium levels and platelets counts during your treatment with NASCOBAL.
The most common side effects of NASCOBAL include:
- infection
- headache
- feeling weak
- nausea
- swelling of your tongue
- runny nose
- tingling of the hands and feet
These are not all of the possible side effects of NASCOBAL.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of NASCOBAL.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use NASCOBAL for a condition for which it was not prescribed. Do not give NASCOBAL to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about NASCOBAL that is written for health professionals.
What are the ingredients in NASCOBAL?
Active ingredient: cyanocobalamin
Inactive ingredients: benzalkonium chloride in purified water, citric acid, glycerin and sodium citrate.
Manufactured by:
Par Pharmaceuticals, Inc.
1 Ram Ridge Road
Chestnut Ridge, NY 10977
For more information, go to www.parpharm.com or call 1-800-828-9393.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 11/2018
-
INSTRUCTIONS FOR USE
NASCOBAL (NAZ. co. bal)
(cyanocobalamin) nasal spray
NASCOBAL is for use in your nose only.
Read this Instructions for Use before you start using NASCOBAL (cyanocobalamin) nasal spray and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or your treatment.
Your healthcare provider should teach you how to use NASCOBAL before you use it for the first time. If you have any questions or do not understand the instructions, talk with your healthcare provider or pharmacist.
NASCOBAL spray contains only 1 spray. You do not need to prime NASCOBAL before you use it.
If you are eating hot foods or liquids, use NASCOBAL at least one hour before or one hour afterwards because eating hot food or drinking hot liquids too soon before or after taking NASCOBAL can cause you to have a runny nose and can affect the amount of NASCOBAL you receive.
How to use NASCOBAL?
Step 1. Blow your nose gently to clear both nostrils.
Step 2. Hold NASCOBAL with your thumb on the bottom and your index (pointer) finger and middle finger on either side of the nozzle (see Figure A).
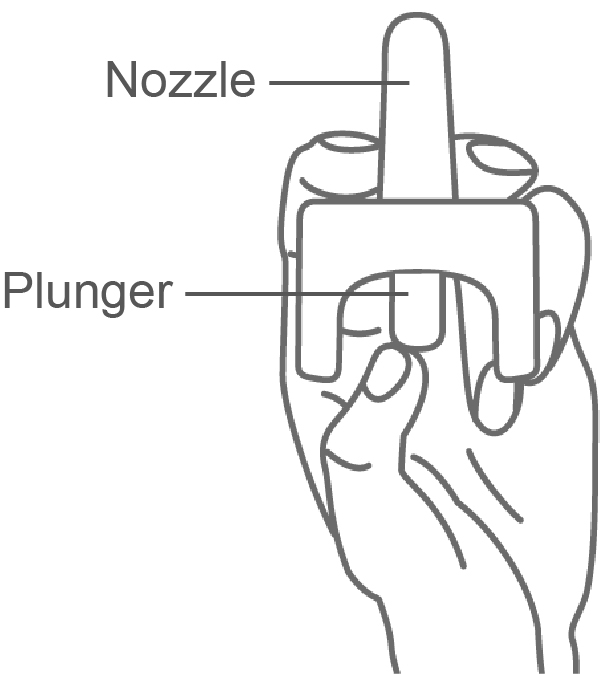
(Figure A)
Step 3. Gently close 1 side of your nose (nostril) with your other index finger. Insert the nozzle into the open nostril about half an inch or as far as it feels comfortable and tilt your head slightly forward (see Figure B). Do not press the plunger yet.
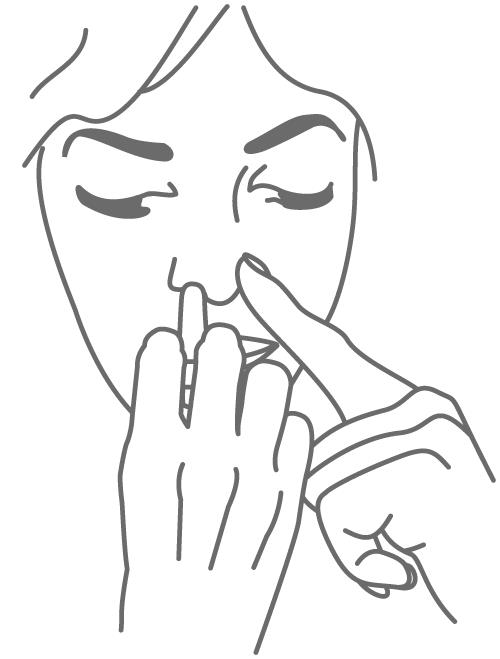
(Figure B)
Step 4. Breathe in gently through your nose, close your mouth, and at the same time press the plunger firmly upwards with your thumb.
Step 5. Remove the nozzle from your nostril. At the same time, keep your head straight for 10 to 20 seconds while gently breathing in through your nose and breathing out through your mouth.
How to dispose of (throw away) NASCOBAL?
NASCOBAL can be disposed of in your household trash. Place the NASCOBAL nasal spray in a container such as a zip-top or sealable plastic bag, and throw the container away in your household trash.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Revised: 11/2018
- PACKAGE LABEL
-
INGREDIENTS AND APPEARANCE
NASCOBAL
cyanocobalamin sprayProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 49884-270 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 500 ug Inactive Ingredients Ingredient Name Strength SODIUM CITRATE (UNII: 1Q73Q2JULR) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) GLYCERIN (UNII: PDC6A3C0OX) BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 49884-270-52 1 in 1 CARTON; Type 0: Not a Combination Product 08/13/2009 04/16/2015 2 NDC: 49884-270-82 4 in 1 CARTON; Type 0: Not a Combination Product 08/13/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021642 08/13/2009 Labeler - Par Pharmaceutical, Inc. (092733690) Establishment Name Address ID/FEI Business Operations Par Pharmaceutical Inc. 092733690 MANUFACTURE(49884-270)
Trademark Results [Nascobal]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NASCOBAL 75194046 2157683 Live/Registered |
ENDO PHARMACEUTICALS INC. 1996-11-06 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.