PHENAZOPYRIDINE HYDROCHLORIDE tablet
Phenazopyridine Hydrochloride by
Drug Labeling and Warnings
Phenazopyridine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Northwind Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
CAUTION: Federal law prohibits dispensing without prescription.
DESCRIPTION
Phenazopyridine Hydrochloride is light or dark red to dark violet, odorless, slightly bitter, crystalline powder. It has a specific local analgesic effect in the urinary tract, promptly relieving burning and pain. Phenazopyridine HCl tablets contain the following inactive ingredients: carnauba wax, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, pregelatinized starch.
-
CLINICAL PHARMACOLOGY
Phenazopyridine HCl is excreted in the urine where it exerts a topical analgesic effect on the mucosa of the urinary tract. This action helps to relieve pain, burning, urgency and frequency. The precise mechanism of action is not known.
The pharmacokinetic properties of Phenazopyridine HCl have not been determined. Phenazopyridine HCl is rapidly excreted by the kidneys, with as much as 66% of an oral dose being excreted unchanged in the urine.
-
INDICATIONS AND USAGE
Phenazopyridine HCl is indicated for the symptomatic relief of pain, burning, urgency, frequency, and other discomforts arising from irritation of the lower urinary tract mucosa caused by infection, trauma, surgery, endoscopic procedures, or the passage of sounds or catheters. The use of Phenazopyridine HCl for relief of symptoms should not delay definitive diagnosis and treatment of causative conditions. Because it provides only symptomatic relief, prompt appropriate treatment of the cause of pain must be instituted and Phenazopyridine HCl should be discontinued when symptoms are controlled.
The analgesic action may reduce or eliminate the need for systemic analgesics or narcotics. It is, however, compatible with antibacterial therapy and can help to relieve pain and discomfort during the interval before antibacterial therapy controls the infection. Treatment of a urinary tract infection with Phenazopyridine HCl should not exceed 2 days because there is a lack of evidence that the combined administration of Phenazopyridine HCl and an antibacterial provides greater benefit than administration of the antibacterial alone after 2 days. (See DOSAGE AND ADMINISTRATION section.)
- CONTRAINDICATIONS
- ADVERSE REACTIONS
-
PRECAUTIONS
General: A yellowish tinge of the skin or sclera may indicate accumulation due to impaired renal excretion and the need to discontinue therapy. The decline in renal function associated with advanced age should be kept in mind.
NOTE: Patients should be informed that Phenazopyridine HCl produces a reddish-orange discoloration of the urine and may stain fabric. Staining of contact lenses has been reported.
Laboratory Test Interaction: Due to its properties as an azo dye, Phenazopyridine HCl may interfere with urinalysis based on spectrometry or color reactions.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term administration of Phenazopyridine HCl has induced neoplasia in rats (large intestine) and mice (liver).
Although no association between Phenazopyridine HCl and human neoplasia has been reported, adequate epidemiological studies along these lines have not been conducted.
Pregnancy Category B: Reproduction studies have been performed in rats at doses up to
50 mg/kg/day and have revealed no evidence of impaired fertility or harm to the fetus due to Phenazopyridine HCl. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing mothers: No information is available on the appearance of Phenazopyridine HCl, or its metabolites in human milk.
-
DOSAGE AND ADMINISTRATION
100 mg Tablets: Average adult dosage is two tablets 3 times a day after meals.
200 mg Tablets: Average adult dosage is one tablet 3 times a day after meals.
When used concomitantly with an antibacterial agent for the treatment of a urinary tract infection, the administration of Phenazopyridine HCl should not exceed 2 days.
-
OVERDOSAGE
Exceeding the recommended dose in patients with good renal function or administering the usual dose to patients with impaired renal function (common in elderly patients) may lead to increased serum levels and toxic reactions. Methemoglobinemia generally follows a massive, acute overdose. Methylene blue, 1 to 2 mg/kg/body weight intravenously or ascorbic acid 100 to 200 mg given orally should cause prompt reduction of the methemoglobinemia and disappearance of the cyanosis which is an aid in diagnosis. Oxidative Heinz body hemolytic anemia may also occur, and “bite cells” (degmacytes) may be present in a chronic overdosage situation. Red blood cell G-6-PD deficiency may predispose to hemolysis. Renal and hepatic impairment and occasional failure, usually due to hypersensitivity, may also occur.
-
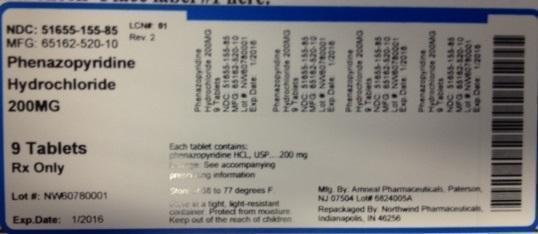
LABEL
NDC: 51655-155-85
MFG: 65162-520-10
Phenazopyridine Hydrochloride 200 MG
9 Tablets
Rx Only
Lot#
Exp Date:
Each tablet contains phenazopyridine HCL, USP 200mg
Dosage: See accompanying prescribing information
Store at 68 to 77 degrees F
Store in a tight, light-resistant container. Protect from moisture.
Keep out of the reach of children.
Mfg by: Amneal Pharmaceuticals, Paterson NJ 07504 Lot#
Repackaged by Northwind Pharmaceuticals, Indianapolis, IN 46256
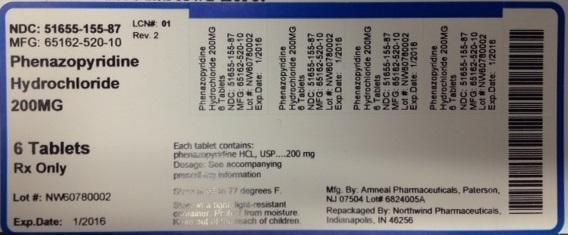
NDC: 51655-155-87
MFG: 65162-520-10
Phenazopyridine Hydrochloride 200 MG
6 Tablets
Rx Only
Lot#
Exp Date:
Each tablet contains phenazopyridine HCL, USP 200mg
Dosage: See accompanying prescribing information
Store at 68 to 77 degrees F
Store in a tight, light-resistant container. Protect from moisture.
Keep out of the reach of children.
Mfg by: Amneal Pharmaceuticals, Paterson NJ 07504 Lot#
Repackaged by Northwind Pharmaceuticals, Indianapolis, IN 46256
-
INGREDIENTS AND APPEARANCE
PHENAZOPYRIDINE HYDROCHLORIDE
phenazopyridine hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 51655-155(NDC:65162-520) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENAZOPYRIDINE HYDROCHLORIDE (UNII: 0EWG668W17) (PHENAZOPYRIDINE - UNII:K2J09EMJ52) PHENAZOPYRIDINE HYDROCHLORIDE 200 mg Product Characteristics Color brown Score no score Shape ROUND Size 10mm Flavor Imprint Code AN;2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51655-155-85 9 in 1 BOTTLE, DISPENSING Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 07/24/2014 Labeler - Northwind Pharmaceuticals, LLC (036986393) Registrant - Northwind Pharmaceuticals, LLC (036986393) Establishment Name Address ID/FEI Business Operations Northwind Pharmaceuticals, LLC 036986393 repack(51655-155)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.