DIFLORASONE DIACETATE cream
Diflorasone Diacetate by
Drug Labeling and Warnings
Diflorasone Diacetate by is a Prescription medication manufactured, distributed, or labeled by E. FOUGERA & CO., A division of Fougera Pharmaceuticals Inc., Fougera Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Diflorasone diacetate cream, 0.05% contains the active compound diflorasone diacetate, a synthetic corticosteroid for topical dermatological use.
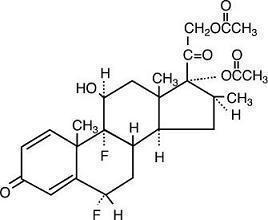
Chemically, diflorasone diacetate is 6α,9-difluoro-11β,17,21-trihydroxy-16β-methylpregna-1,4-diene-3,20-dione 17,21-diacetate, with the molecular formula C26H32F2O7, a molecular weight of 494.54, and the following structural formula:
Each gram of diflorasone diacetate cream, 0.05% contains: 0.5 mg diflorasone diacetate in a cream base consisting of cetyl alcohol, glyceryl stearate SE (nonionic), isopropyl myristate, mineral oil (and) lanolin alcohol, monobasic sodium phosphate, monoglyceride citrate, polyoxyl 40 stearate, polysorbate 60, propylene glycol, purified water, sorbitan monostearate, vegetable oil, butylated hydroxytoluene and citric acid.
-
CLINICAL PHARMACOLOGY
Like other topical corticosteroids, diflorasone diacetate has anti-inflammatory, anti-pruritic, and vasoconstrictive actions. The mechanism of the anti-inflammatory activity of the topical corticosteroids, in general, is unclear. However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor, arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
Pharmacokinetics: The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle and the integrity of the epidermal barrier. Occlusive dressings with hydrocortisone for up to 24 hours have not been demonstrated to increase penetration; however, occlusion of hydrocortisone for 96 hours markedly enhances penetration. Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin may increase percutaneous absorption. Studies performed with diflorasone diacetate cream indicate that it is in the high range of potency as compared with other topical corticosteroids.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General: Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment. Manifestations of Cushing's syndrome, hyperglycemia, and glucosuria can also be produced in some patients by systemic absorption of topical corticosteroids while on treatment.
Patients receiving a large dose of a higher potency topical steroid applied to a large surface area or under an occlusive dressing should be evaluated periodically for evidence of HPA axis suppression. This may be done by using the ACTH-stimulation, A.M. plasma cortisol, and urinary free-cortisol tests.
This product has a greater ability to produce adrenal suppression than does diflorasone diacetate ointment, 0.05%. At 30 g per day (applied as 15 g twice daily) diflorasone diacetate cream, 0.05% was shown to cause inhibition of the HPA axis in one of two patients following application for one week to psoriatic skin. At 15 g per day (applied as 7.5 g twice daily) diflorasone diacetate cream was shown to cause mild inhibition of the HPA axis in one of five patients following application for one week to diseased skin (psoriasis or atopic dermatitis). These effects were reversible upon discontinuation of treatment. By comparison, diflorasone diacetate ointment 0.05% did not produce significant HPA axis suppression when used in divided doses at 30 g per day for one week in patients with psoriasis or atopic dermatitis.
If HPA axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of application, or to substitute a less potent corticosteroid. Recovery of HPA axis function is generally prompt and complete upon discontinuation of topical corticosteroids. Infrequently, signs and symptoms of glucocorticosteroid insufficiency may occur, requiring supplemental systemic corticosteroids. For information on systemic supplementation, see prescribing information for those products.
Children may be more susceptible to systemic toxicity from equivalent doses due to their larger skin surface to body mass ratios (see PRECAUTIONS: Pediatric Use).
If irritation develops, diflorasone diacetate cream should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing failure to heal rather than noting a clinical exacerbation as with most topical products not containing corticosteroids. Such an observation should be corroborated with appropriate diagnostic patch testing.
If concomitant skin infections are present or develop, an appropriate antifungal or antibacterial agent should be used. If a favorable response does not occur promptly, use of diflorasone diacetate cream should be discontinued until the infection has been adequately controlled.
Diflorasone diacetate cream should not be used in the treatment of rosacea or perioral dermatitis, and it should not be used on the face, groin, or axillae.
Information for Patients: Patients using topical corticosteroids should receive the following information and instructions:
- The medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
- The medication should not be used for any disorder other than that for which it was prescribed.
- The treated skin area should not be bandaged or otherwise covered or wrapped so as to be occlusive unless directed by the physician.
- Patients should report to their physician any signs of local adverse reactions.
Laboratory Tests: The following tests may be helpful in evaluating patients for HPA axis suppression: ACTH-stimulation test; A.M. plasma-cortisol test; Urinary free-cortisol test.
Carcinogenesis, Mutagenesis, Impairment of Fertility: Long-term animal studies have not been performed to evaluate the carcinogenic potential of diflorasone diacetate.
Diflorasone diacetate was not found to be mutagenic in a micronucleus test in rats at dosages of 2400 mg/kg.
Studies in the rat following topical administration at doses up to 0.5 mg/kg revealed no effects on fertility.
Pregnancy: Teratogenic effects– Pregnancy Category C. Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application to laboratory animals.
Diflorasone diacetate has been shown to be teratogenic (cleft palate) in rats when applied topically at a dose of approximately 0.001 mg/kg/day to the shaven thorax of pregnant animals. This is approximately 0.3 times the human topical dose of diflorasone diacetate cream. When pregnant rats were treated topically with approximately 0.5 mg/kg/day, uterine deaths were higher in the treated animals than in control animals.
In rabbits, cleft palate was seen when diflorasone diacetate was applied in topical doses as low as 20 mg/kg/day. In addition, fetal weight was depressed and litter sizes were smaller.
There are no adequate and well-controlled studies of the teratogenic potential of diflorasone diacetate in pregnant women. Diflorasone diacetate cream should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers: Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when diflorasone diacetate cream is administered to a nursing woman.
Pediatric Use: Safety and effectiveness of diflorasone diacetate cream in pediatric patients have not been established. Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA-axis suppression when they are treated with topical corticosteroids. They are, therefore, also at greater risk of glucocorticosteroid insufficiency after withdrawal of treatment and of Cushing's syndrome while on treatment. Adverse effects including striae have been reported with inappropriate use of topical corticosteroids in infants and pediatric patients.
HPA axis suppression, Cushing's syndrome, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids. Manifestations of adrenal suppression in pediatric patients include linear growth retardation, delayed weight gain, low plasma cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
-
ADVERSE REACTIONS
The following local adverse reactions have been reported infrequently with other topical corticosteroids, and they may occur more frequently with the use of occlusive dressings, especially with higher potency corticosteroids. These reactions are listed in an approximate decreasing order of occurrence: burning, itching, irritation, dryness, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infections, skin atrophy, striae, and miliaria.
-
OVERDOSAGE
Topically applied diflorasone diacetate cream, can be absorbed in sufficient amounts to produce systemic effects (see PRECAUTIONS).
- DOSAGE AND ADMINISTRATION
-
HOW SUPPLIED
Diflorasone Diacetate Cream USP, 0.05% is available as follows:
NDC: 0168-0242-15 15 gram tube
NDC: 0168-0242-30 30 gram tube
NDC: 0168-0242-60 60 gram tubeStore at or below 25°C (77°F). Keep tightly closed.
E. FOUGERA & CO.
a division of Fougera Pharmaceuticals Inc.
Melville, New York 11747I242B
R03/12
#171 -
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 15 G CONTAINER
NDC: 0168-0242-15
Fougera®
DIFLORASONE DIACETATE
CREAM USP, 0.05%
FOR EXTERNAL USE ONLY
NOT FOR OPHTHALMIC USE
Rx only
Each gram contains: 0.5 mg
diflorasone diacetate in a cream base
consisting of cetyl alcohol, glyceryl
stearate SE (nonionic), isopropyl
myristate, mineral oil (and) lanolin,
alcohol, monobasic sodium phosphate,
monoglyceride citrate, polyoxyl 40
stearate, polysorbate 60, propylene
glycol, purified water, sorbitan
monostearate, vegetable oil, butylated
hydroxytoluene and citric acid.NET WT 15 grams
-
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – 15 G CARTON
NDC: 0168-0242-15
Fougera® Rx only
DIFLORASONE DIACETATE
CREAM USP, 0.05%
FOR EXTERNAL USE ONLY
NOT FOR OPHTHALMIC USE
WARNING: Keep out of reach
of children.
NET WT 15 grams
-
INGREDIENTS AND APPEARANCE
DIFLORASONE DIACETATE
diflorasone diacetate creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0168-0242 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIFLORASONE DIACETATE (UNII: 7W2J09SCWX) (DIFLORASONE - UNII:T2DHJ9645W) DIFLORASONE DIACETATE 0.5 mg in 1 g Inactive Ingredients Ingredient Name Strength CETYL ALCOHOL (UNII: 936JST6JCN) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) MINERAL OIL (UNII: T5L8T28FGP) LANOLIN ALCOHOLS (UNII: 884C3FA9HE) SODIUM PHOSPHATE, MONOBASIC (UNII: 3980JIH2SW) POLYOXYL 40 STEARATE (UNII: 13A4J4NH9I) POLYSORBATE 60 (UNII: CAL22UVI4M) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SORBITAN MONOSTEARATE (UNII: NVZ4I0H58X) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0168-0242-15 15 g in 1 TUBE 2 NDC: 0168-0242-30 30 g in 1 TUBE 3 NDC: 0168-0242-60 60 g in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075187 03/30/1998 Labeler - E. FOUGERA & CO., A division of Fougera Pharmaceuticals Inc. (043838424) Establishment Name Address ID/FEI Business Operations Fougera Pharmaceuticals Inc. 174491316 MANUFACTURE(0168-0242) Establishment Name Address ID/FEI Business Operations Fougera Pharmaceuticals Inc. 043838424 ANALYSIS(0168-0242)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.