CORALITE PAIN RELIEF- camphor, menthol, methyl salicylate patch
Coralite Pain Relief by
Drug Labeling and Warnings
Coralite Pain Relief by is a Otc medication manufactured, distributed, or labeled by United Exchange Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
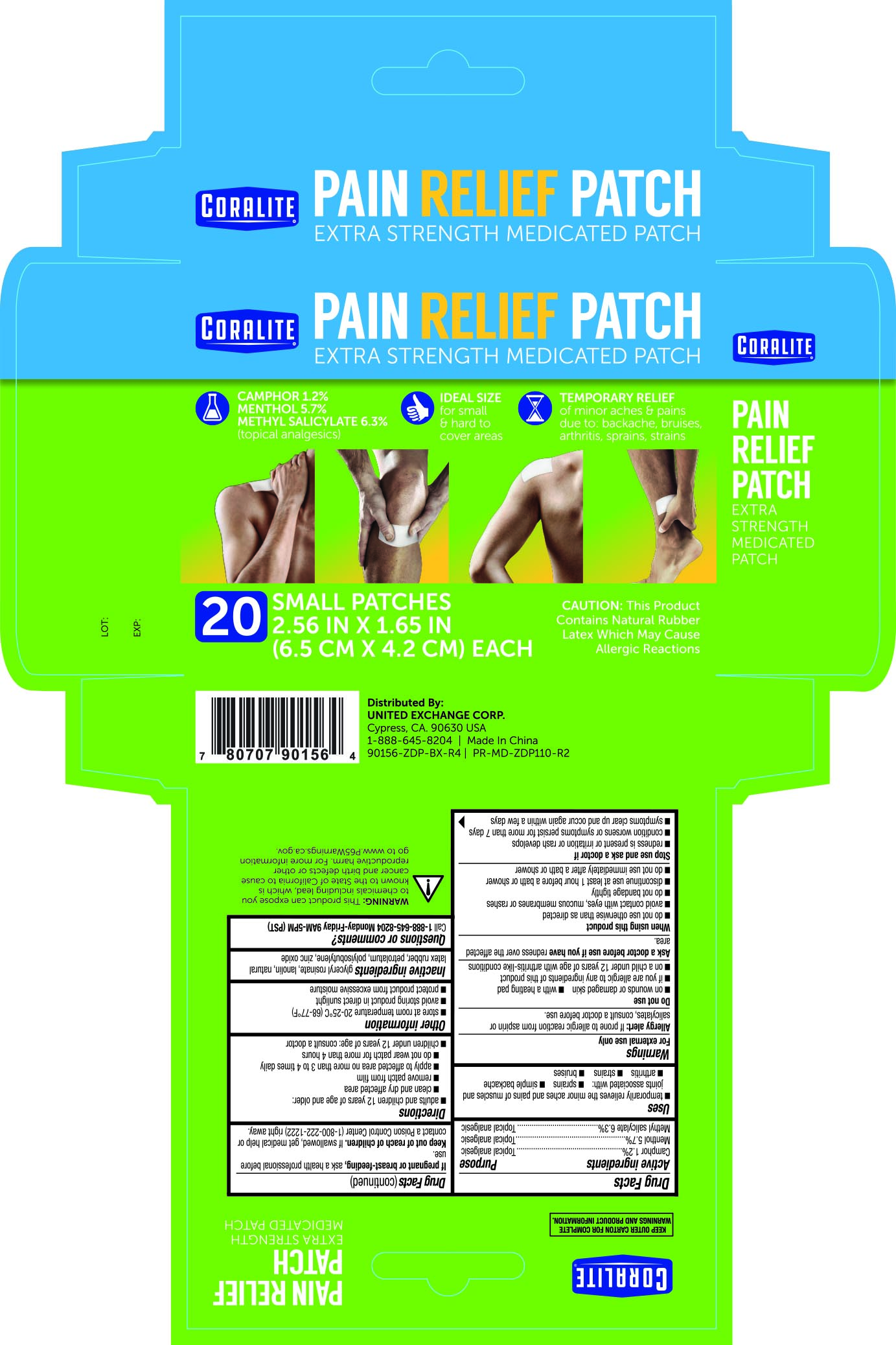
- ACTIVE INGREDIENT
- PURPOSE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- WHEN USING
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- STORAGE AND HANDLING
- INACTIVE INGREDIENT
- DOSAGE & ADMINISTRATION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CORALITE PAIN RELIEF
camphor, menthol, methyl salicylate patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 65923-564 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CAMPHOR (SYNTHETIC) (UNII: 5TJD82A1ET) (CAMPHOR (SYNTHETIC) - UNII:5TJD82A1ET) CAMPHOR (SYNTHETIC) 1.2 g in 100 g MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 5.7 g in 100 g METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 6.3 g in 100 g Inactive Ingredients Ingredient Name Strength LANOLIN (UNII: 7EV65EAW6H) NATURAL LATEX RUBBER (UNII: 2LQ0UUW8IN) PETROLATUM (UNII: 4T6H12BN9U) ZINC OXIDE (UNII: SOI2LOH54Z) POLYISOBUTYLENE (800000 MW) (UNII: Y132ZOQ9H7) GLYCERYL ROSINATE (UNII: SD112V492J) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 65923-564-20 20 in 1 PACKAGE 04/12/2018 1 1 g in 1 PATCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 04/12/2016 Labeler - United Exchange Corp. (840130579)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.