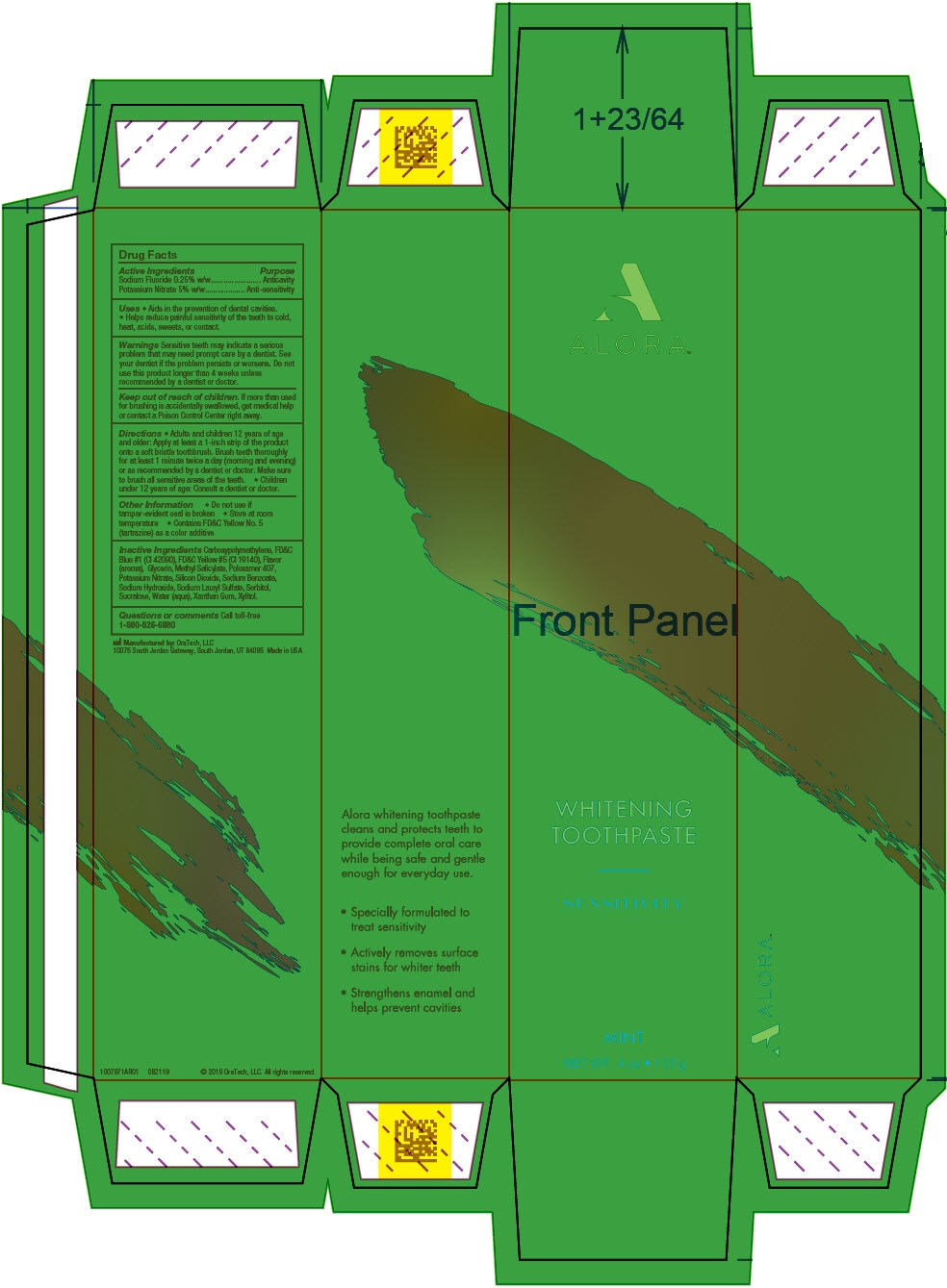
ALORA SENSITIVITY- potassium nitrate and sodium fluoride gel, dentifrice
Alora by
Drug Labeling and Warnings
Alora by is a Otc medication manufactured, distributed, or labeled by Amazon, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- Uses
- Warnings
-
Directions
- Adults and children 12 years of age and older: Apply at least a 1-inch strip of the product onto a soft bristle toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist or doctor. Make sure to brush all sensitive areas of the teeth.
- Children under 12 years of age: Consult a dentist or doctor.
- Other Information
- Inactive Ingredients
- Questions or comments
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 113 g Tube Carton
-
INGREDIENTS AND APPEARANCE
ALORA SENSITIVITY
potassium nitrate and sodium fluoride gel, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72288-309 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Potassium Nitrate (UNII: RU45X2JN0Z) (Nitrate Ion - UNII:T93E9Y2844) Potassium Nitrate 50 mg in 1 g Sodium Fluoride (UNII: 8ZYQ1474W7) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 2.5 mg in 1 g Inactive Ingredients Ingredient Name Strength XYLITOL (UNII: VCQ006KQ1E) Water (UNII: 059QF0KO0R) Silicon Dioxide (UNII: ETJ7Z6XBU4) GLYCERIN (UNII: PDC6A3C0OX) SORBITOL (UNII: 506T60A25R) Poloxamer 407 (UNII: TUF2IVW3M2) Methyl Salicylate (UNII: LAV5U5022Y) Sodium Lauryl Sulfate (UNII: 368GB5141J) Xanthan Gum (UNII: TTV12P4NEE) Sodium Benzoate (UNII: OJ245FE5EU) CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) Sodium Hydroxide (UNII: 55X04QC32I) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Sucralose (UNII: 96K6UQ3ZD4) Product Characteristics Color GREEN Score Shape Size Flavor MINT (Cool Mint) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72288-309-02 3 in 1 PACKAGE, COMBINATION 10/01/2019 1 NDC: 72288-309-01 1 in 1 CARTON 1 28.35 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC: 72288-309-03 6 in 1 PACKAGE, COMBINATION 10/01/2019 2 NDC: 72288-309-01 1 in 1 CARTON 2 28.35 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part356 10/01/2019 Labeler - Amazon, Inc (128990418)
Trademark Results [Alora]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALORA 98558762 not registered Live/Pending |
Alora Health, Inc. 2024-05-20 |
 ALORA 98558759 not registered Live/Pending |
Alora Health, Inc. 2024-05-20 |
 ALORA 98083237 not registered Live/Pending |
Willow Wolfe Inc. 2023-07-13 |
 ALORA 98001065 not registered Live/Pending |
AVB Hospitality LLC 2023-05-17 |
 ALORA 97546625 not registered Live/Pending |
Sage Industrial LLC 2022-08-12 |
 ALORA 97244586 not registered Live/Pending |
Kuzco Lighting LLC 2022-01-28 |
 ALORA 97244579 not registered Live/Pending |
Kuzco Lighting LLC 2022-01-28 |
 ALORA 97014423 not registered Live/Pending |
Mejia, Orlana 2021-09-07 |
 ALORA 90815658 not registered Live/Pending |
Nutrition Distribution LLC 2021-07-07 |
 ALORA 90075532 not registered Live/Pending |
Alora TM, LLC 2020-07-27 |
 ALORA 88380670 5951792 Live/Registered |
Scherr, Samuel R 2019-04-10 |
 ALORA 88318333 not registered Live/Pending |
Oratech, LLC 2019-02-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.