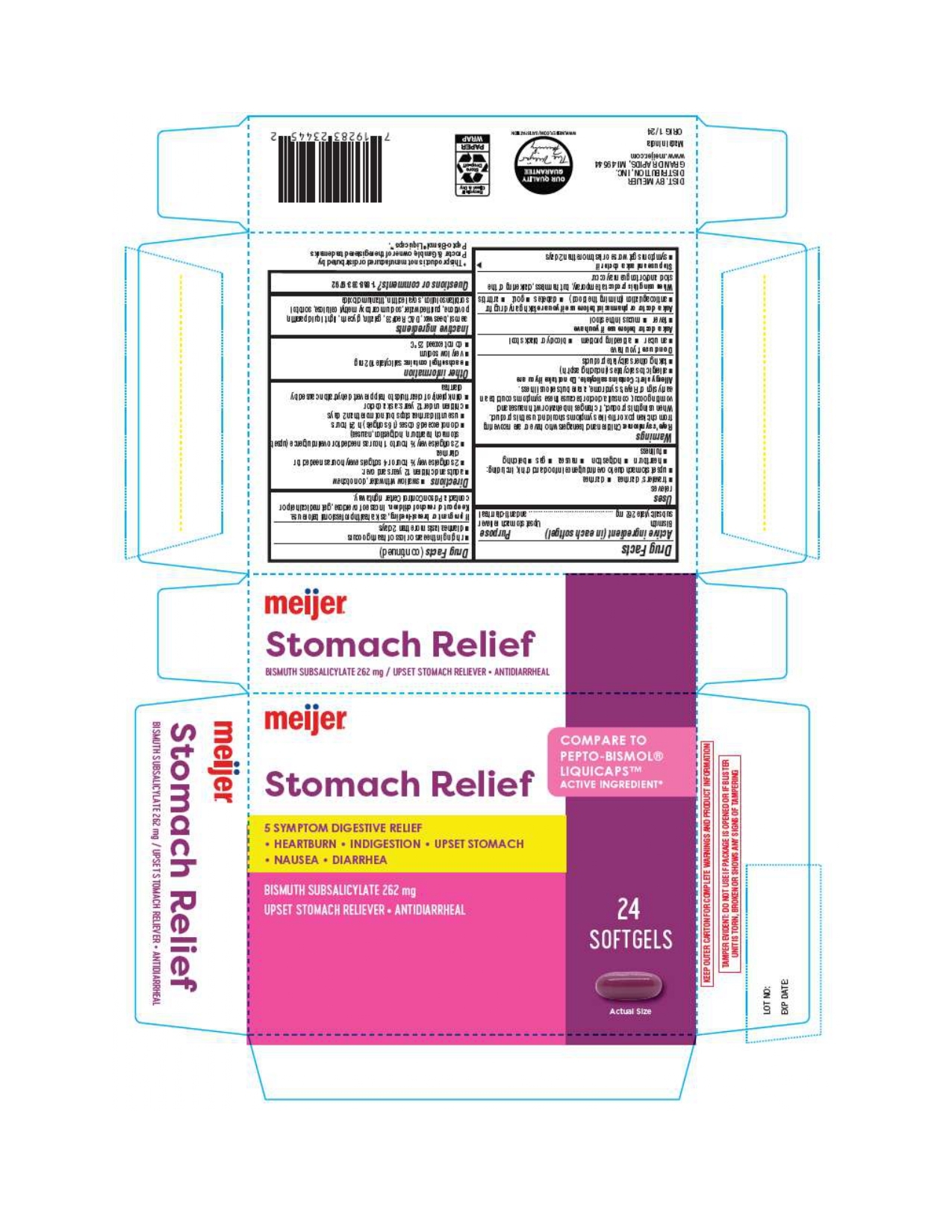
Bismuth Subsalicylate by MEIJER DISTRIBUTION, INC. Stomach Relief
Bismuth Subsalicylate by
Drug Labeling and Warnings
Bismuth Subsalicylate by is a Otc medication manufactured, distributed, or labeled by MEIJER DISTRIBUTION, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BISMUTH SUBSALICYLATE- bismuth subsalicylate capsule, liquid filled
MEIJER DISTRIBUTION, INC.
----------
Stomach Relief
Uses
relieves
travelers' diarrhea diarrhea
upset stomach due to overindulgence in food and drink, including:
heartburn indigestion nausea gas belching fullness
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product.
When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an
early sign of Reye’s syndrome, a rare but serious illness.
Allergy alert: Contains salicylate. Do not take if you are
allergic to salicylates (including aspirin)
taking other salicylate products
Ask a doctor or pharmacist before use if you are taking any drug for
anticoagulation (thinning the blood) diabetes gout arthritis
Stop use and ask a doctor if
symptoms get worse or last more than 2 days
ringing in the ears or loss of hearing occurs
diarrhea lasts more than 2 days
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
swallow with water, do not chew
adults and children 12 years and over:
2 softgels every ½ hour or 4 softgels every hour as needed for
diarrhea
2 softgels every ½ hour to 1 hour as needed for overindulgence (upset
stomach, heartburn, indigestion, nausea)
do not exceed 8 doses (16 softgels) in 24 hours
use until diarrhea stops but not more than 2 days
children under 12 years: ask a doctor
drink plenty of clear fluids to help prevent dehydration caused by diarrhea
| BISMUTH SUBSALICYLATE
bismuth subsalicylate capsule, liquid filled |
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||
| Labeler - MEIJER DISTRIBUTION, INC. (006959555) |
Revised: 12/2024
Document Id: 29badfc9-38e4-f310-e063-6294a90a8790
Set id: 69ee3cf9-b31b-4a02-8fef-dea31ec27506
Version: 3
Effective Time: 20241220