VALCHLOR- mechlorethamine hydrochloride gel
VALCHLOR by
Drug Labeling and Warnings
VALCHLOR by is a Prescription medication manufactured, distributed, or labeled by Helsinn Therapeutics (U.S.), Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use VALCHLOR ® safely and effectively. See full prescribing information for VALCHLOR.
VALCHLOR (mechlorethamine) gel, for topical use
Initial U.S. Approval: 1949INDICATIONS AND USAGE
VALCHLOR is an alkylating drug indicated for the topical treatment of Stage IA and IB mycosis fungoides-type cutaneous T-cell lymphoma in patients who have received prior skin-directed therapy ( 1).
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Gel: 0.016% w/w of mechlorethamine (equivalent to 0.02% mechlorethamine HCl) in 60g tubes ( 3)
CONTRAINDICATIONS
Severe hypersensitivity to mechlorethamine ( 4)
WARNINGS AND PRECAUTIONS
- Mucosal or eye injury: VALCHLOR exposure to mucous membranes, especially of the eyes, can cause mucosal injury which may be severe. Eye injury may lead to blindness. Immediately irrigate for at least 15 minutes followed by immediate medical consultation ( 5.1).
- Secondary exposure to VALCHLOR: individuals other than the patient must avoid skin contact with VALCHLOR ( 2.2, 5.2).
- Dermatitis: Monitor patients for redness, swelling, inflammation, itchiness, blisters, ulceration, and secondary skin infections. Stop treatment or reduce dose frequency ( 2.1, 5.3).
- Non-melanoma skin cancer: Monitor patients during and after treatment ( 5.4).
- Embryo-fetal toxicity: May cause fetal harm ( 5.5).
- Flammable gel: VALCHLOR is an alcohol-based gel. Avoid fire, flame, and smoking until the gel has dried ( 2.2, 5.6).
ADVERSE REACTIONS
The most common adverse reactions (≥5%) are dermatitis, pruritus, bacterial skin infection, skin ulceration or blistering, and hyperpigmentation ( 6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Helsinn Therapeutics (U.S.), Inc., at 1-855-482-5245 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.USE IN SPECIFIC POPULATIONS
Lactation: Advise not to breastfeed ( 8.2).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 1/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosing and Dose Modification
2.2 Application Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Mucosal or Eye Injury
5.2 Secondary Exposure to VALCHLOR
5.3 Dermatitis
5.4 Non-Melanoma Skin Cancer
5.5 Embryo-fetal Toxicity
5.6 Flammable Gel
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosing and Dose Modification
For Topical Dermatological Use Only
Apply a thin film of VALCHLOR gel once daily to affected areas of the skin.
Stop treatment with VALCHLOR for any grade of skin ulceration, blistering, or moderately-severe or severe dermatitis (i.e., marked skin redness with edema) [ see Warnings and Precautions ( 5.3) ]. Upon improvement, treatment with VALCHLOR can be restarted at a reduced frequency of once every 3 days. If reintroduction of treatment is tolerated for at least one week, the frequency of application can be increased to every other day for at least one week and then to once daily application if tolerated.
2.2 Application Instructions
VALCHLOR is a cytotoxic drug. Follow applicable special handling and disposal procedures. 1
Patients must wash hands thoroughly with soap and water after handling or applying VALCHLOR.
Caregivers must wear disposable nitrile gloves when applying VALCHLOR to patients and wash hands thoroughly with soap and water after removal of gloves. If there is accidental skin exposure to VALCHLOR, caregivers must immediately wash exposed areas thoroughly with soap and water for at least 15 minutes and remove contaminated clothing [ see Warnings and Precautions ( 5.2) ].
Patients or caregivers should follow these instructions when applying VALCHLOR:
- Apply immediately or within 30 minutes after removal from the refrigerator. Return VALCHLOR to the refrigerator immediately after each use.
- Apply to completely dry skin at least 4 hours before or 30 minutes after showering or washing. Allow treated areas to dry for 5 to 10 minutes after application before covering with clothing.
- Emollients (moisturizers) may be applied to the treated areas 2 hours before or 2 hours after application.
- Do not use occlusive dressings on areas of the skin where VALCHLOR was applied.
- Avoid fire, flame, and smoking until VALCHLOR has dried [ see Warnings and Precautions ( 5.6) ].
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Mucosal or Eye Injury
Exposure of the eyes to mechlorethamine causes pain, burns, inflammation, photophobia, and blurred vision. Blindness and severe irreversible anterior eye injury may occur. Advise patients that if eye exposure occurs, (1) immediately irrigate for at least 15 minutes with copious amounts of water, normal saline, or a balanced salt ophthalmic irrigating solution and (2) obtain immediate medical care (including ophthalmologic consultation).
Exposure of mucous membranes such as the oral mucosa or nasal mucosa causes pain, redness, and ulceration, which may be severe. Should mucosal contact occur, immediately irrigate for at least 15 minutes with copious amounts of water, followed by immediate medical consultation.
5.2 Secondary Exposure to VALCHLOR
Avoid direct skin contact with VALCHLOR in individuals other than the patient. Risks of secondary exposure include dermatitis, mucosal injury, and secondary cancers. Follow recommended application instructions to prevent secondary exposure [ see Dosage and Administration ( 2.2) ].
5.3 Dermatitis
The most common adverse reaction was dermatitis, which occurred in 56% of the patients [ see Adverse Reactions ( 6.1) ]. Dermatitis was moderately severe or severe in 23% of patients. Monitor patients for redness, swelling, inflammation, itchiness, blisters, ulceration, and secondary skin infections. The face, genitalia, anus, and intertriginous skin are at increased risk of dermatitis. Follow dose modification instructions for dermatitis [ see Dosage and Administration ( 2.1) ].
5.4 Non-Melanoma Skin Cancer
Four percent (4%, 11/255) of patients developed a non-melanoma skin cancer during the clinical trial or during one year of post-treatment follow-up: 2% (3/128) of patients receiving VALCHLOR, and 6% (8/127) of patients receiving the mechlorethamine ointment comparator. Some of these non-melanoma skin cancers occurred in patients who had received prior therapies known to cause non-melanoma skin cancer. Monitor patients for non-melanoma skin cancers during and after treatment with VALCHLOR. Non-melanoma skin cancer may occur on any area of the skin, including untreated areas.
5.5 Embryo-fetal Toxicity
Based on case reports in humans, findings in animal reproduction studies, its mechanism of action, and genotoxicity findings, mechlorethamine may cause fetal harm. There are case reports of children born with malformations in pregnant women systemically administered mechlorethamine. Mechlorethamine was teratogenic and embryo-lethal after a single subcutaneous administration to animals. Advise women to avoid becoming pregnant while using VALCHLOR. If this drug is used during pregnancy or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to a fetus [ see Use in Specific Populations ( 8.1) ].
5.6 Flammable Gel
Alcohol-based products, including VALCHLOR, are flammable. Follow recommended application instructions [ see Dosage and Administration ( 2.2) ].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are discussed in greater detail in other sections of the prescribing information:
- Mucosal or eye injury [ see Warnings and Precautions ( 5.1) ]
- Secondary exposure to VALCHLOR [ see Warnings and Precautions ( 5.2) ]
- Dermatitis [ see Warnings and Precautions ( 5.3) ]
- Non-melanoma skin cancer [ see Warnings and Precautions ( 5.4) ]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In a randomized, observer-blinded, controlled trial, VALCHLOR 0.016% (equivalent to 0.02% mechlorethamine HCl) was compared to an Aquaphor ®-based mechlorethamine HCl 0.02% ointment (Comparator) [ see Clinical Studies ( 14) ]. The maximum duration of treatment was 12 months. Sixty-three percent (63%) of patients in the VALCHLOR arm and 67% in the comparator arm completed 12 months of treatment.
The body system associated with the most frequent adverse reactions was skin and subcutaneous tissue disorders. The most common adverse reactions (occurring in at least 5% of the patients) are shown in Table 1.
Table 1. Most Commonly Reported (≥5%) Cutaneous Adverse Reactions VALCHLOR
N=128
% of patientsComparator
N=127
% of patientsAny Grade Moderately-Severe
or SevereAny Grade Moderately-Severe
or SevereDermatitis 56 23 58 17 Pruritus 20 4 16 2 Bacterial skin infection 11 2 9 2 Skin ulceration or blistering 6 3 5 2 Skin hyperpigmentation 5 0 7 0 In the clinical trial, moderately-severe to severe skin-related adverse events were managed with treatment reduction, suspension, or discontinuation. Discontinuations due to adverse reactions occurred in 22% of patients treated with VALCHLOR and 18% of patients treated with the comparator. Sixty-seven percent (67%) of the discontinuations for adverse reactions occurred within the first 90 days of treatment. Temporary treatment suspension occurred in 34% of patients treated with VALCHLOR and 20% of patients treated with the comparator. Reductions in dosing frequency occurred in 23% of patients treated with VALCHLOR and 12% of patients treated with the comparator.
Reductions in hemoglobin, neutrophil count, or platelet count occurred in 13% of patients treated with VALCHLOR and 17% treated with Comparator.
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on case reports in humans, findings in animal reproduction studies, its mechanism of action, and genotoxicity findings, mechlorethamine may cause fetal harm.
Available published case reports in pregnant women receiving intravenous mechlorethamine demonstrate that mechlorethamine can cause major birth defects when a pregnant woman is systemically exposed. In animal reproduction studies, subcutaneous administration of mechlorethamine to pregnant rats and ferrets during organogenesis resulted in embryo‐fetal mortality, alterations to growth, and structural abnormalities. Based on limited available data with VALCHLOR use in pregnant women, if VALCHLOR is used during pregnancy or if the patient becomes pregnant while taking this drug, patient should be advised of the potential risk to the fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
The limited available data with VALCHLOR use in pregnant women does not show evidence of congenital malformation in newborns. Cases of newborns with congenital malformations have been reported in women who received systemic mechlorethamine during pregnancy.Animal Data
Mechlorethamine caused fetal malformations in the rat and ferret when given as single subcutaneous injections of 1 mg/kg. Other findings in animals included embryo lethality and growth retardation when administered as a single subcutaneous injection.8.2 Lactation
Risk Summary
There are no data on the presence of mechlorethamine or its metabolites in human milk, the effects of the drug on the breastfed child, or the effects of the drug on milk production. Because of the potential for topical or systemic exposure to VALCHLOR through exposure to the mother's skin and the potential for serious adverse reactions in the breastfed child from mechlorethamine, advise patients not to breastfeed during treatment with VALCHLOR.
8.3 Females and Males of Reproductive Potential
Contraception
Females
Advise female patients of reproductive potential to use effective contraception during treatment with VALCHLOR. A barrier method of contraception should be used to avoid direct exposure of reproductive organs to VALCHLOR.
Males
Based on genotoxicity findings, advise males with female partners of reproductive potential to use effective contraception during treatment with VALCHLOR [ see Nonclinical Toxicology ( 13.1) ]. A barrier method of contraception should be used to avoid direct exposure of reproductive organs to VALCHLOR.
Infertility
Based on animal data, mechlorethamine may impair fertility in males and females [ see Nonclinical Toxicology ( 13.1) ]. The reversibility of the effect on fertility is unknown.
8.5 Geriatric Use
A total of 79 patients age 65 and older (31% of the clinical trial population) were treated with either VALCHLOR or the comparator in the clinical trial. Forty-four percent (44%) of patients age 65 or older treated with VALCHLOR achieved a CAILS response compared to 66% of patients below the age of 65. Seventy percent (70%) of patients age 65 and older experienced cutaneous adverse reactions and 38% discontinued treatment due to adverse reactions, compared to 58% and 14% in patients below the age of 65, respectively. Similar differences in discontinuation rates between age subgroups were observed in the comparator group.
-
11 DESCRIPTION
VALCHLOR is a topical product that contains mechlorethamine HCl, an alkylating drug. Mechlorethamine HCl is a white to off white solid that is very soluble in water and methanol, partially soluble in acetone, and generally not soluble in organic solvents.
Mechlorethamine HCl is designated chemically as 2-chloro- N-(2-chloroethyl)- N-methylethanamine hydrochloride. The molecular weight is 192.52 and the melting point is 108-111°C. The empirical formula is C 5H 11Cl 2NHCl, and the structural formula is: CH 3N(CH 2CH 2Cl) 2HCl.
Each tube of VALCHLOR contains 60g of a gel containing 0.016% w/w of mechlorethamine (equivalent to 0.02% mechlorethamine HCl) in a base of the following inactive ingredients: diethylene glycol monoethyl ether, propylene glycol, isopropyl alcohol, glycerin, lactic acid, hydroxypropylcellulose, sodium chloride, menthol, edetate disodium, butylated hydroxytoluene.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Mechlorethamine, also known as nitrogen mustard, is an alkylating agent which inhibits rapidly proliferating cells.
12.3 Pharmacokinetics
Systemic exposure was undetectable after topical administration of VALCHLOR to patients. Blood samples were analyzed from 16 and 15 patients following treatment with VALCHLOR (mechlorethamine gel 0.016%) and an identical formulation consisting of mechlorethamine 0.032% w/w, respectively. For patients who received mechlorethamine 0.016%, samples were collected to measure mechlorethamine concentrations prior to dosing, on day 1, and at the first month visit. Following the topical administration of mechlorethamine 0.016%, there were no detectable plasma mechlorethamine concentrations observed in any of the patients. Patients who received mechlorethamine 0.032% had no measurable concentrations of mechlorethamine or half-mustard after 2, 4, or 6 months of treatment.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Mechlorethamine was carcinogenic in mice when injected intravenously with four doses of 2.4 mg/kg (0.1% solution) at 2-week intervals with observations for up to 2 years. An increased incidence of thymic lymphomas and pulmonary adenomas was observed. Painting mechlorethamine on the skin of mice at a dose of 4 mg/kg for periods of up to 33 weeks resulted in squamous cell tumors in 9 of 33 mice.
Mechlorethamine was genotoxic in multiple genetic toxicology studies, which included mutations in the bacterial reverse mutation assay (Ames test) and chromosome aberrations in mammalian cells. Dominant lethal mutations were produced in ICR/Ha Swiss mice.
The reproductive effects of VALCHLOR have not been studied; however, published literature indicates that fertility may be impaired by systemically administered mechlorethamine. Mechlorethamine impaired fertility in the male rats at a daily dose of 0.25 to 0.5 mg/kg when given intravenously every two weeks for up to 12 doses. When mechlorethamine was administered intraperitoneally to male and female mice for 4 consecutive days at a dose of 0.5 mg/kg the pregnancy rate decreased (from 80% to 12.5%) when treated males were paired with treated females. Treatment with intravenous mechlorethamine has been associated with delayed catamenia, oligomenorrhea, and temporary or permanent amenorrhea.
-
14 CLINICAL STUDIES
The efficacy of VALCHLOR was assessed in a randomized, multicenter, observer-blind, active-controlled, non-inferiority clinical trial of 260 patients with Stage IA, IB, and IIA mycosis fungoides-type cutaneous T-cell lymphoma (MF-CTCL) who had received at least one prior skin-directed therapy. Qualifying prior therapies included topical corticosteroids, phototherapy, Targretin ® gel, and topical nitrogen mustard. Patients were not required to be refractory to or intolerant of prior therapies.
Patients were stratified based on Stage (IA vs. IB and IIA) and then randomized to receive VALCHLOR 0.016% (equivalent to 0.02% mechlorethamine HCl) or Aquaphor ®-based mechlorethamine HCl 0.02% ointment (Comparator) at 13 centers in the United States. Eighteen patients were excluded from the efficacy analysis due to protocol violations involving randomization at a single site.
Study drug was to be applied topically on a daily basis for 12 months. Concomitant use of topical corticosteroids was not permitted during the study. Dosing could be suspended or continued with reduced frequency for dermatitis. The mean daily usage of VALCHLOR gel was 2.8 g (1 to 2 tubes per month). The maximum daily usage was 10.5 g (5 to 6 tubes per month).
Patients were evaluated for a response on a monthly basis for the first 6 months and then every 2 months for the last 6 months using the Composite Assessment of Index Lesion Severity (CAILS) score. The CAILS score is obtained by adding the severity score of each of the following categories for up to 5 index lesions: erythema, scaling, plaque elevation, and surface area. Severity was graded from 0 (none) to 8 (severe) for erythema and scaling; 0 to 3 for plaque elevation; and 0 to 9 for surface area. A response was defined as greater than or equal to 50% reduction in baseline CAILS score which was confirmed at the next visit at least 4 weeks later. A complete response was defined as a confirmed CAILS score of 0. Non-inferiority was considered to have been demonstrated if the lower bound of the 95% confidence interval (CI) around the ratio of response rates (VALCHLOR/Comparator) was greater than or equal to 0.75.
Patients were also evaluated using the Severity Weighted Assessment Tool (SWAT). The SWAT score is derived by measuring each involved area as a percentage of total body surface area (%BSA) and multiplying it by a severity weighting factor (1=patch, 2=plaque, 3=tumor or ulcer). A response was defined as greater than or equal to 50% reduction in baseline SWAT score which was confirmed at the next visit at least 4 weeks later.
The baseline demographics and disease characteristics were balanced between treatment arms. The median age was 57 years in the VALCHLOR arm and 58 years in the comparator arm. The majority of the patients were male (60% in VALCHLOR arm, 59% in Comparator arm) and white (75% in both treatment arms). The median number of prior therapies was 2 in both treatment arms. The most common prior therapy was topical corticosteroids (used in 86% of patients in both treatment arms). The median body surface area (BSA) involvement at baseline was 8.5% (range 1%, 61%) in the VALCHLOR arm and 9% (range 1%, 76%) in the comparator arm.
Sixty percent (60%) of the patients on the VALCHLOR arm and 48% of patients on the comparator arm achieved a response based on the CAILS score. VALCHLOR was non-inferior to the comparator based on a CAILS overall response rate ratio of 1.24 (95% CI 0.98, 1.58). Complete responses constituted a minority of the CAILS or SWAT overall responses ( Table 2). The onset of CAILS overall response for both treatment arms showed a wide range from 1 to 11 months.
Table 2. Efficacy in Patients with Mycosis Fungoides-Type Cutaneous T-Cell Lymphoma (MF-CTCL) Response Rates VALCHLOR
N=119Comparator
N=123CAILS Overall Response (CR+PR)
Complete Response (CR)
Partial Response (PR)60%
14%
45%48%
11%
37%SWAT Overall Response (CR+PR)
Complete Response (CR)
Partial Response (PR)50%
7%
43%46%
3%
43% - 15 REFERENCES
- 16 HOW SUPPLIED/STORAGE AND HANDLING
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling ( Medication Guide)
Advise patients of the following and provide a copy of the Medication Guide.
Instructions for Patients and Caregivers for Application of VALCHLOR:
Apply a thin film of VALCHLOR once daily to affected areas of the skin [ see Dosage and Administration ( 2.1) ].Patients must wash hands thoroughly with soap and water after handling or applying VALCHLOR. Caregivers must wear disposable nitrile gloves when applying VALCHLOR to patients and wash hands thoroughly with soap and water after removal of gloves. If there is accidental skin exposure to VALCHLOR, caregivers must immediately wash exposed areas thoroughly with soap and water and remove contaminated clothing [ see Dosage and Administration ( 2.2) ].
Patients and caregivers should follow these instructions when applying VALCHLOR [ see Dosage and Administration ( 2.2) ]:
- Apply immediately or within 30 minutes after removal from the refrigerator. Return VALCHLOR to the refrigerator immediately after each use.
- Apply VALCHLOR to completely dry skin at least 4 hours before or 30 minutes after showering or washing. Allow treated areas to dry for 5 to 10 minutes after application before covering with clothing.
- Emollients (moisturizers) may be applied to the treated areas 2 hours before or 2 hours after application of VALCHLOR.
- Occlusive (air or water-tight) dressings should not be used on areas of the skin where VALCHLOR was applied.
Instructions for Patients and Caregivers for Storage of VALCHLOR
Store VALCHLOR refrigerated at temperatures between 36°F - 46°F (2°C - 8°C). Advise patients that adherence to the recommended storage condition will ensure VALCHLOR will work as expected. Patients should consult a pharmacist prior to using VALCHLOR that has been left at room temperature for longer than one hour per day. Unused product should be discarded after 90 days [ see How Supplied/Storage and Handling ( 16) ].With clean hands, replace tube in the original box, then place in the refrigerator. Keep VALCHLOR in its original box out of the reach of children and avoid contact with food when storing in the refrigerator.
Unused VALCHLOR, empty tubes, and used application gloves should be discarded in household trash in a manner that prevents accidental application or ingestion by others, including children and pets.
Mucosal or Eye Injury
Exposure of the eyes to mechlorethamine causes pain, burns, inflammation, photophobia, and blurred vision. Blindness and severe irreversible eye injury may occur. Should eye contact occur, immediately irrigate for at least 15 minutes with copious amounts of water, normal saline, or a balanced salt ophthalmic irrigating solution, followed by immediate ophthalmologic consultation [ see Warnings and Precautions ( 5.1) ].Exposure of mucous membranes such as the oral mucosa or nasal mucosa causes pain, redness, and ulceration, which may be severe. Should mucosal contact occur, immediately irrigate for at least 15 minutes with copious amounts of water, followed by immediate medical consultation [ see Warnings and Precautions ( 5.1) ].
Secondary Exposure to VALCHLOR
Avoid direct skin contact with VALCHLOR in individuals other than the patient. Risks of secondary exposure include dermatitis, mucosal injury, and secondary cancers. Caregivers who help apply VALCHLOR to patients must wear disposable nitrile gloves when handling VALCHLOR. If secondary exposure occurs to eyes, mouth, or nose, immediately irrigate the exposed area for at least 15 minutes with copious amounts of water. Thoroughly wash affected areas of the skin with soap and water [ see Dosage and Administration ( 2.2) and Warnings and Precautions ( 5.2) ].Dermatitis
If patients experience skin irritation after applying VALCHLOR, such as redness, swelling, inflammation, itchiness, blisters, ulceration, or secondary skin infections, instruct patients to discuss with their physician options for changes in the treatment plan. The face, genitalia, anus, or intertriginous skin (skin folds or creases) are at increased risk of skin irritation [ see Warnings and Precautions ( 5.3) ].Non-Melanoma Skin Cancers
Instruct patients to notify their physician of any new skin lesions and to undergo periodic assessment for signs and symptoms of skin cancer. Non-melanoma skin cancers have been reported in patients receiving the active ingredient in VALCHLOR. Non-melanoma skin cancer may occur at multiple areas, including areas not directly treated with VALCHLOR [ see Warnings and Precautions ( 5.4) ].Embryo-fetal Toxicity
Advise women of the potential risk to the fetus and to avoid pregnancy while using VALCHLOR. Advise males with female partners of reproductive potential to use a barrier method of contraception while using VALCHLOR [ see Use in Specific Populations ( 8.1, 8.3) ].
Lactation
Advise females not to breastfeed during treatment with VALCHLOR [ see Use in Specific Populations ( 8.2) ].
Manufactured for:
Helsinn Therapeutics, (U.S.), Inc., Iselin, NJ 08830 -
MEDICATION GUIDE
MEDICATION GUIDE
VALCHLOR ® (val-klor)
(mechlorethamine) gelImportant information: VALCHLOR is for use on skin only. Do not get VALCHLOR near or in your eyes, mouth, or nose. What is the most important information I should know about VALCHLOR?
Keep VALCHLOR away from your eyes, mouth, and nose.- If VALCHLOR gets in your eyes it can cause eye pain, burning, swelling, redness, sensitivity to light, and blurred vision. It may also cause blindness and permanent injury to your eyes. If VALCHLOR gets in your eyes, rinse your eyes right away for at least 15 minutes with a large amount of water, normal saline, or an eye wash solution.
- If VALCHLOR gets in your mouth or nose it can cause pain, redness, and ulcers. Rinse the affected area right away for at least 15 minutes with a large amount of water.
Get medical help right away if VALCHLOR gets in your eyes, mouth, or nose. Talk with your healthcare provider before using VALCHLOR about how to get medical help.
What is VALCHLOR?
VALCHLOR is a prescription medicine used on the skin (topical) to treat people with Stage 1A and 1B mycosis fungoides-type cutaneous T-cell lymphoma who have received previous skin treatment. It is not known if VALCHLOR is safe and effective in children.Do not use VALCHLOR if you are severely allergic to mechlorethamine. Tell your healthcare provider if you have had an allergic reaction to mechlorethamine. Before using VALCHLOR, tell your healthcare provider about all of your medical conditions, including if you:
- are pregnant or plan to become pregnant. VALCHLOR may harm your unborn baby. Avoid becoming pregnant during treatment with VALCHLOR. Tell your healthcare provider right away if you become pregnant while using VALCHLOR.
-Females who are able to become pregnant should use a barrier method of birth control, such as a male condom or spermicide, during treatment with VALCHLOR.
-Males with female partners who are able to become pregnant should use a barrier method of birth control, such as a male condom or spermicide, during treatment with VALCHLOR.
- are breastfeeding or plan to breastfeed. It is not known if VALCHLOR passes into your breast milk. Do not breastfeed during treatment with VALCHLOR.
Tell your healthcare provider about all the medicines you take including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use VALCHLOR? - Use VALCHLOR exactly as your healthcare provider tells you.
- Caregivers must wear disposable nitrile gloves when applying VALCHLOR.
- Wash your hands with soap and water after touching or applying VALCHLOR.
- Safely throw away used disposable nitrile gloves in household trash.
- Apply VALCHLOR right away or within 30 minutes after you take it out of the refrigerator.
- Return VALCHLOR to the refrigerator right after each use.
- Apply a thin layer of VALCHLOR 1 time each day (or as instructed by your healthcare providers) to completely dry affected areas of the skin at least 4 hours before or 30 minutes after showering or washing.
- Let the treated areas dry for 5 to 10 minutes after applying VALCHLOR before covering with clothing.
- Moisturizers may be applied to the treated areas 2 hours before or 2 hours after applying VALCHLOR.
- You should not use air or water-tight bandages on areas of the skin treated with VALCHLOR.
What should I avoid while using VALCHLOR?
VALCHLOR is flammable. Avoid fire, flame, and smoking until VALCHLOR has dried.What are the possible side effects of VALCHLOR?
See " What is the most important information I should know about VALCHLOR?"
VALCHLOR can cause serious side effects, including:-
Risk of Secondary Exposure to VALCHLOR. You may have a risk of inflammation of your skin (dermatitis), injury to your eyes, mouth, or nose, and certain types of cancers. Caregivers who accidentally come into contact with VALCHLOR must wash the affected area with soap and water right away for at least 15 minutes and remove any contaminated clothing.
Get medical help right away if VALCHLOR gets in your eyes, mouth, or nose. - Inflammation of your skin (dermatitis) is common with VALCHLOR and may sometimes be severe. Your risk for dermatitis is increased if VALCHLOR is applied to your face, genital area, anus, or skin folds. Tell your healthcare provider if you develop skin reactions such as redness, swelling, itching, blisters, ulcers, and skin infections.
- Increased risk of certain types of skin cancers. Certain types of skin cancer can develop on areas of your skin that are treated with VALCHLOR and areas of your skin that are not treated with VALCHLOR. Your healthcare provider will check your skin for skin cancers during and after your treatment with VALCHLOR. Tell your healthcare provider if you get any new skin lesions.
The most common side effects of VALCHLOR include: redness, swelling, itching, skin ulcers or blisters, skin infection, and darkening of areas of your skin.
VALCHLOR may cause fertility problems in females and males. This could affect the ability to become pregnant. Talk to your healthcare provider if this is a concern for you.
These are not all the possible side effects of VALCHLOR.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.How should I store VALCHLOR? - Store VALCHLOR in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Keep VALCHLOR away from food in the refrigerator.
- With clean hands, place VALCHLOR back in the box it came in and return it to the refrigerator right after each use.
- Talk with your pharmacist before you use VALCHLOR that has been out of the refrigerator for more than one hour a day.
- Safely throw away VALCHLOR that is not used after 90 days. Unused VALCHLOR, empty tubes, and used disposable nitrile gloves should be safely thrown away in household trash.
- Keep VALCHLOR and all medicines out of the reach of children.
General information about the safe and effective use of VALCHLOR.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use VALCHLOR for a condition for which it was not prescribed. Do not give VALCHLOR to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about VALCHLOR that is written for health professionals.What are the ingredients in VALCHLOR?
Active ingredient: mechlorethamine
Inactive ingredients: diethylene glycol monoethyl ether, propylene glycol, isopropyl alcohol, glycerin, lactic acid, hydroxypropylcellulose, sodium chloride, menthol, edetate disodium, butylated hydroxytoluene
Manufactured for: Helsinn Therapeutics (U.S.), Inc., Iselin, NJ 08830
For more information, go to www.VALCHLOR.com or call 1-855-482-5245.This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 01/2020
-
Principal Display Panel - 60 g Tube Carton
VALCHLOR®
(mechlorethamine) gel
0.016%For Topical Use
Dispense with Medication GuideBefore dispensing, store in freezer
After dispensing, store refrigeratedRX Only
NET WT 60 gramsNDC: 69639-120-01
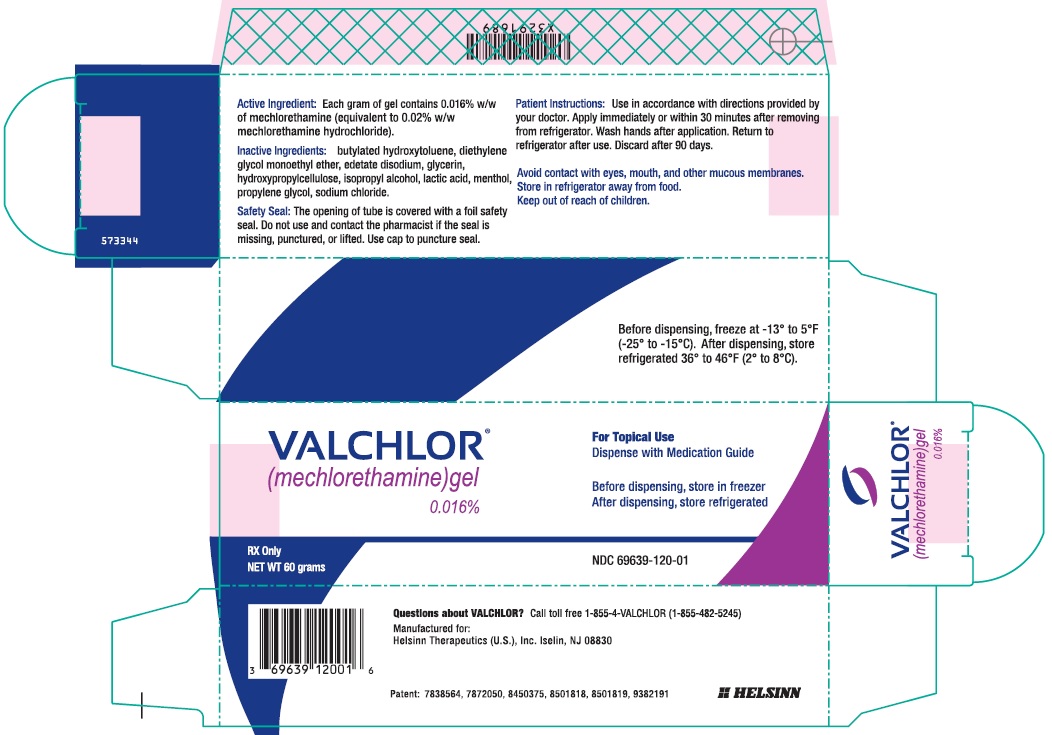
- Tube Label - Front
- Tube Label - Back
-
INGREDIENTS AND APPEARANCE
VALCHLOR
mechlorethamine hydrochloride gelProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69639-120 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MECHLORETHAMINE (UNII: 50D9XSG0VR) (MECHLORETHAMINE - UNII:50D9XSG0VR) MECHLORETHAMINE 0.012 g in 60 g Inactive Ingredients Ingredient Name Strength DIETHYLENE GLYCOL MONOETHYL ETHER (UNII: A1A1I8X02B) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ISOPROPYL ALCOHOL (UNII: ND2M416302) GLYCERIN (UNII: PDC6A3C0OX) LACTIC ACID, UNSPECIFIED FORM (UNII: 33X04XA5AT) HYDROXYPROPYL CELLULOSE (1600000 WAMW) (UNII: RFW2ET671P) SODIUM CHLORIDE (UNII: 451W47IQ8X) RACEMENTHOL (UNII: YS08XHA860) EDETATE DISODIUM (UNII: 7FLD91C86K) BUTYLATED HYDROXYTOLUENE (UNII: 1P9D0Z171K) Product Characteristics Color white (clear) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69639-120-01 1 in 1 CARTON 11/08/2018 1 60 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA202317 11/08/2018 Labeler - Helsinn Therapeutics (U.S.), Inc. (829472583)
Trademark Results [VALCHLOR]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VALCHLOR 85425856 4538181 Live/Registered |
HELSINN BIREX PHARMACEUTICALS LIMITED 2011-09-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

