MEDERMA- avobenzone, octocrylene, and oxybenzone cream
Mederma by
Drug Labeling and Warnings
Mederma by is a Otc medication manufactured, distributed, or labeled by Merz Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- DOSAGE & ADMINISTRATION
- Active Ingredients
-
Inactive Ingredients
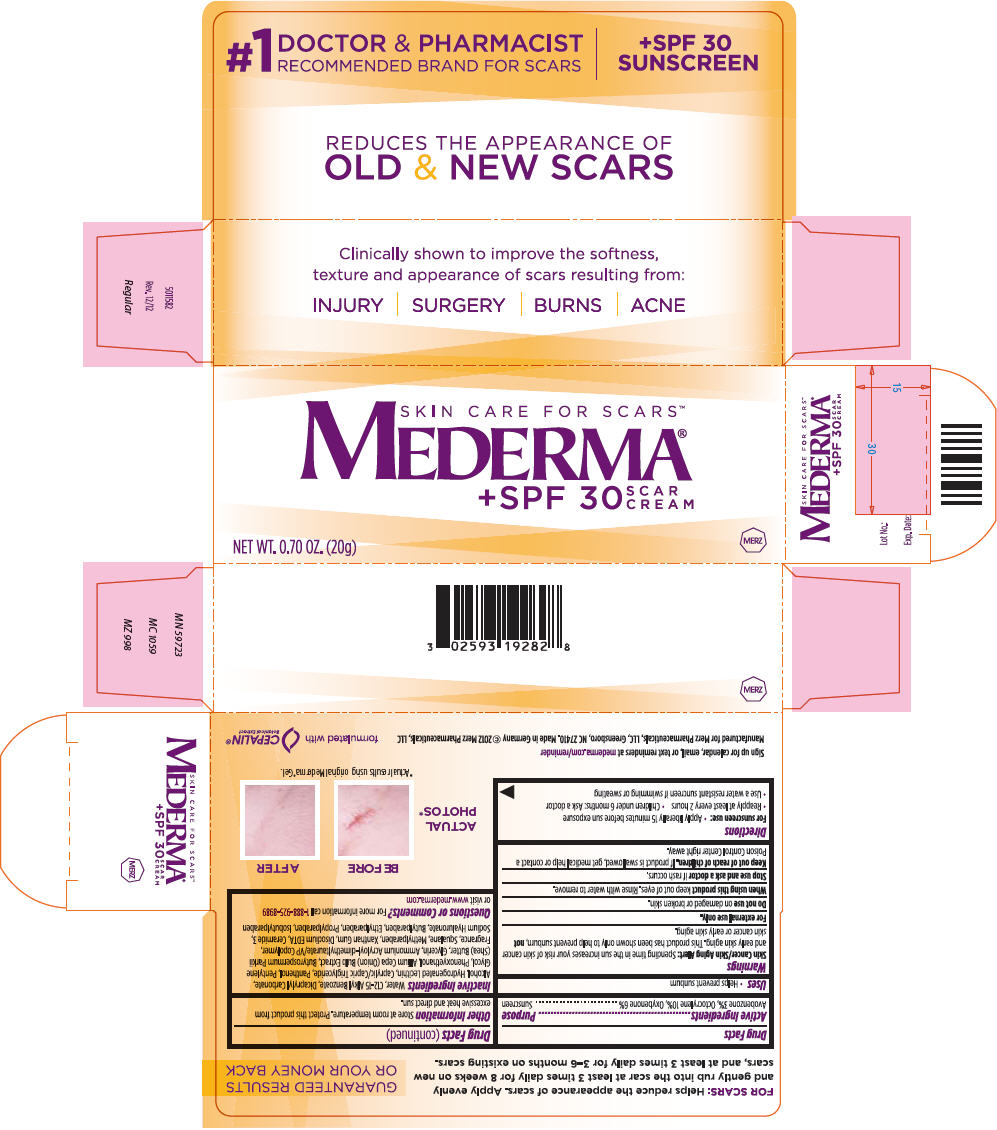
WATER, ALLIUM CEPA (ONION) BULB EXTRACT, C12-15 ALKYL BENZOATE, DICAPRYLYL CARBONATE, HYDROGENATED LECITHIN, CAPRYLIC/CAPRIC TRIGLYCERIDE, PANTHENOL, PENTYLENE GLYCOL, PHENOXYETHANOL, BUTYROSPERMUM PARKII (SHEA BUTTER), GLYCERIN, AMMONIUM ACRYLOYL-DIMETHYLTAURATE/VP COPOLYMER, FRAGRANCE, SQUALANE, METHYLPARABEN, XANTHAN GUM, DISODIUM EDTA, CERAMIDE 3, SODIUM HYALURONATE, BUTYLPARABEN, ETHYLPARABEN, PROPYLPARABEN, ISOBUTYLPARABEN.
- Warnings
- STORAGE AND HANDLING
- QUESTIONS
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 20 g Tube Carton
-
INGREDIENTS AND APPEARANCE
MEDERMA
avobenzone, octocrylene, and oxybenzone creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0259-3192 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 30 mg in 1 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 100 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 60 mg in 1 g Inactive Ingredients Ingredient Name Strength ONION (UNII: 492225Q21H) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) PANTHENOL (UNII: WV9CM0O67Z) PENTYLENE GLYCOL (UNII: 50C1307PZG) PHENOXYETHANOL (UNII: HIE492ZZ3T) SHEA BUTTER (UNII: K49155WL9Y) GLYCERIN (UNII: PDC6A3C0OX) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) SQUALANE (UNII: GW89575KF9) METHYLPARABEN (UNII: A2I8C7HI9T) XANTHAN GUM (UNII: TTV12P4NEE) EDETATE DISODIUM (UNII: 7FLD91C86K) CERAMIDE NP (UNII: 4370DF050B) HYALURONATE SODIUM (UNII: YSE9PPT4TH) BUTYLPARABEN (UNII: 3QPI1U3FV8) ETHYLPARABEN (UNII: 14255EXE39) PROPYLPARABEN (UNII: Z8IX2SC1OH) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) C12-15 ALCOHOLS (UNII: 2C8M6XLB5C) ALCOHOL (UNII: 3K9958V90M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0259-3192-05 0.5 g in 1 PACKET; Type 0: Not a Combination Product 12/01/2007 2 NDC: 0259-3192-20 1 in 1 CARTON 12/01/2007 2 20 g in 1 TUBE; Type 0: Not a Combination Product 3 NDC: 0259-3192-50 1 in 1 CARTON 12/01/2007 3 50 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part352 12/01/2007 Labeler - Merz Pharmaceuticals, LLC (126209282)
Trademark Results [Mederma]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MEDERMA 98837601 not registered Live/Pending |
Laboratoire HRA Pharma SAS 2024-11-05 |
 MEDERMA 98837577 not registered Live/Pending |
Laboratoire HRA Pharma SAS 2024-11-05 |
 MEDERMA 98835009 not registered Live/Pending |
Laboratoire HRA Pharma SAS 2024-11-04 |
 MEDERMA 88933830 not registered Live/Pending |
LABORATOIRE HRA PHARMA 2020-05-26 |
 MEDERMA 79403136 not registered Live/Pending |
Laboratoire HRA Pharma SAS 2024-07-09 |
 MEDERMA 78908193 3233153 Live/Registered |
LABORATOIRE HRA PHARMA 2006-06-14 |
 MEDERMA 77907204 4084438 Live/Registered |
LABORATOIRE HRA PHARMA 2010-01-07 |
 MEDERMA 75179474 2464771 Live/Registered |
LABORATOIRE HRA PHARMA 1996-10-10 |
 MEDERMA 74696233 1981076 Dead/Cancelled |
MERZ, INCORPORATED 1995-07-03 |
 MEDERMA 74696128 2360460 Live/Registered |
LABORATOIRE HRA PHARMA 1995-07-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.