CALCIUM CHLORIDE injection
Calcium Chloride by
Drug Labeling and Warnings
Calcium Chloride by is a Prescription medication manufactured, distributed, or labeled by Medical Purchasing Solutions, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
Calcium Chloride Injection, USP, 10%, is a sterile aqueous solution containing, in each mL, 100 mg (1.36 mEq) calcium chloride. The pH of the solution may have been adjusted with hydrochloric acid and / or calcium hydroxide, when necessary. The air above the liquid in the individual containers has been displaced by flushing with nitrogen during the filling operation. The preparation contains no antimicrobial preservatives and is intended as a single-dose vial; once the unit is assembled and used, any remaining portion of the solution must be discarded with the entire unit.
Calcium Chloride, USP, contains two molecules of water of hydration and is chemically designated as CaCl 2 2H 20.
-
CLINICAL PHARMACOLOGY
Calcium is the fifth most abundant element in the body, the major fraction of which is found in the bony structure. Calcium plays important physiological roles; it is essential for the functional integrity of the nervous and muscular systems; it is necessary for normal cardiac function; and it is one of the factors involved in the mechanism of blood coagulation.
-
INDICATIONS AND USAGE
Calcium Chloride Injection, USP, 10% is indicated:
In the treatment of hypocalcemia in conditions requiring a prompt increase in plasma calcium levels (e.g., neonatal tetany and tetany due to parathyroid deficiency, vitamin D deficiency and alkalosis) and for prevention of hypocalcemia during exchange transfusions.
As adjunctive therapy in the management of acute symptoms in lead colic.
In the treatment of magnesium intoxication due to overdosage of magnesium sulfate.
In severe hyperkalemia, to combat deleterious effects on electrocardiographic (ECG) function, pending correction of the potassium level in the extracellular fluid.
In cardiac resuscitation, particularly after open heart surgery, when epinephrine fails to improve weak or ineffective myocardial contractions.
- CONTRAINDICATIONS
-
WARNINGS
Calcium chloride should be injected into a large vein very slowly, as it may cause peripheral vasodilatation and a cutaneous burning sensation. A moderate fall in blood pressure due to vasodilatation may attend the injection. Since calcium chloride is an acidifying salt, it is usually undesirable in the treatment of hypocalcemia or renal insufficiency.
-
PRECAUTIONS
General
Calcium Chloride Injection, USP, 10% is irritating to veins and must not be injected into tissues, since severe necrosis and sloughing may occur. Great care should be taken to avoid extravasation or accidental injection into perivascular tissues.
Solutions should be warmed to body temperature. Injections should be made slowly through a small needle into a large vein to minimize venous irritation and avoid undesirable reactions. It is particularly important to prevent a high concentration of calcium from reaching the heart because of the danger of cardiac syncope. If injected into the ventricular cavity in cardiac resuscitation care must be taken to avoid injection into the myocardial tissue.
Drug Interactions
Because of the danger involved in the simultaneous use of calcium salts and drugs of the digitalis group, a digitalized patient should not receive an intravenous injection of a calcium compound unless the indications are clearly defined.
Calcium salts should not generally be mixed with carbonates, phosphates, sulfates or tartrates in parenteral admixtures.
ADVERSE REACTIONS
Rapid I.V. injection may cause the patient to complain of tingling sensations, a calcium taste, a sense of oppression or “heat wave.”
Injections of calcium chloride are accompanied by peripheral vasodilation as well as a local “burning” sensation, and there may be a moderate fall in blood pressure.
DOSAGE AND ADMINISTRATION
FOR INTRACARDIAC OR INTRAVENOUS USE ONLYINJECT SLOWLY
Calcium Chloride Injection, USP, 10%, is administered only by slow intravenous injection (not to exceed 1 mL/min) and / or in cardiac resuscitation, by injection into the ventricular cavity. It must not be injected into the myocardium.The usual precautions for intravenous therapy should be observed. If time permits, the solution should be warmed to body temperature. The injection should be halted if the patient complains of any discomfort; it may be resumed when symptoms disappear. Following injection, the patient should remain recumbent for a short time.
INTRACARDIAC USE
For cardiac resuscitation, inject into the ventricular cavity, not into the heart muscle.Usual Adult Dosage: 200 to 800 mg (2 to 8 mL) when injected into the ventricular cavity.
Pediatric Dosage: 0.2 mL/kg of body weight.
INTRAVENOUS USE
Hypocalcemic DisordersUsual Adult Dosage: 500 mg to 1 g (5 to 10 mL) at intervals of 1 to 3 days, depending on the response of the patient and / or results of serum calcium determinations. Repeated injections may be required because of rapid excretion of calcium.
Pediatric Dosage: 0.2 mL /kg of body weight. Maximum 1-10 mL/day.
Magnesium Intoxication
Initial Adult Dose: 500 mg (5 mL) administered promptly and the patient observed for signs of recovery before further doses are given.Hyperkalemic ECG Disturbances of Cardiac Function
Dosage should be adjusted by constant monitoring of ECG changes during administration. -
HOW SUPPLIED
CALCIUM CHLORIDE INJECTION, USP, 10%
In unit-use packages containing a Luer-Jet™ Luer-Lock Prefilled Syringe.
Stock No. 3304 NDC: 76329-3304-1 10 mL
Ten cartons per package.
Syringe Assembly Directions:
USE ASEPTIC TECHNIQUE
Do not assemble until ready to use.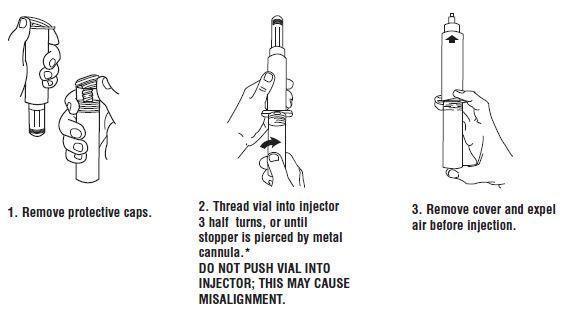
*CAUTION: IMPROPER ENGAGING MAY CAUSE GLASS BREAKAGE AND SUBSEQUENT INJURY.
Store at controlled room temperature 15° to 30°C (59° to 86°F).
Rx Only
INTERNATIONAL MEDICATION SYSTEMS, LIMITEDSo. El Monte, CA 91733, U.S.A. Rev. 2-13
An Amphastar Pharmaceuticals Company© INTERNATIONAL MEDICATION SYSTEMS, LIMITED 2013
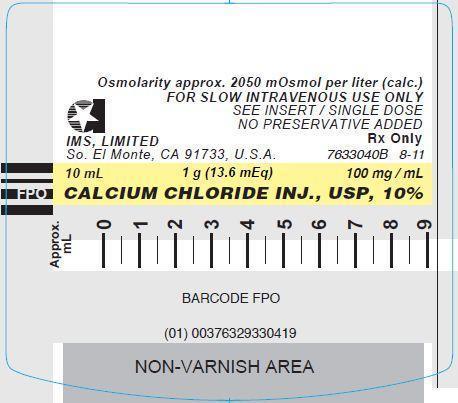
- PRINCIPLE DISPLAY PANEL: Syringe Label
-
PRINCIPLE DISPLAY PANEL: Carton
Luer-Lock Prefilled Syringe
Rx only
NDC: 76329-3304-1
STOCK NO. 3304
CALCIUM CHLORIDE INJ. USP, 10%
1.36 mEq/ mL (100 mg/ mL)
13.6 mEq (1 g) per 10 mL
One 10 mL Single Dose Prefilled Syringe
Single use, do not reuse or resterilize.
FOR SLOW INTRAVENOUS USE
LUER-JET TM LUER-LOCK PREFILLED SYRINGE
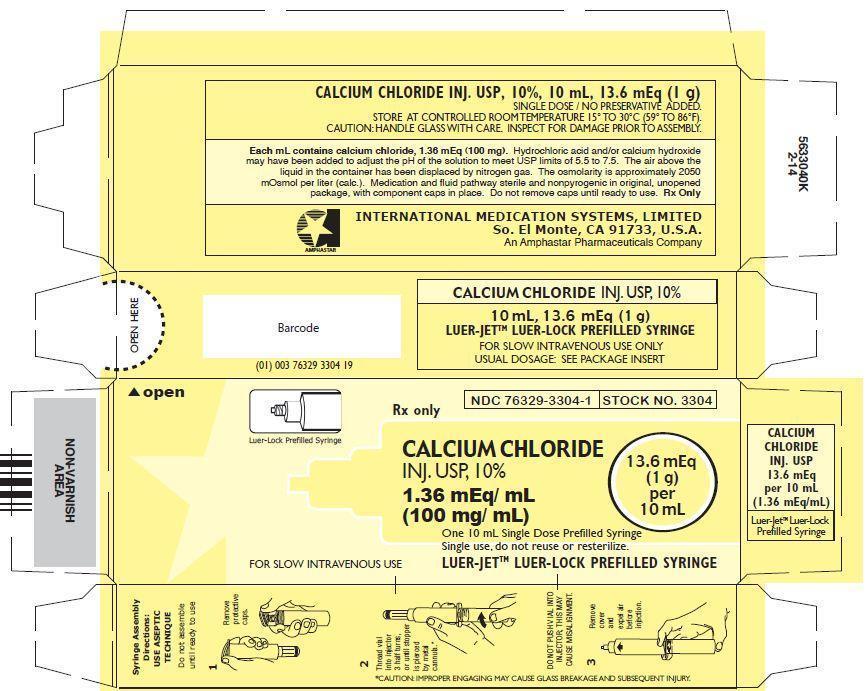
- PRINCIPAL DISPLAY PANEL: Outer package label
-
INGREDIENTS AND APPEARANCE
CALCIUM CHLORIDE
calcium chloride injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 71872-7044(NDC:76329-3304) Route of Administration INTRAVENOUS, INTRAVENTRICULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CHLORIDE (UNII: M4I0D6VV5M) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CHLORIDE 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength HYDROCHLORIC ACID (UNII: QTT17582CB) CALCIUM HYDROXIDE (UNII: PF5DZW74VN) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 71872-7044-1 1 in 1 BAG 03/15/2018 1 1 in 1 CARTON 1 10 mL in 1 SYRINGE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 05/01/1973 Labeler - Medical Purchasing Solutions, LLC (601458529) Establishment Name Address ID/FEI Business Operations Medical Purchasing Solutions, LLC 601458529 repack(71872-7044)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.