MIEBO- perfluorohexyloctane solution
MIEBO by
Drug Labeling and Warnings
MIEBO by is a Prescription medication manufactured, distributed, or labeled by Bausch & Lomb Incorporated, Alliance Medical Products, Inc. (dba Siegfried Irvine). Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use MIEBO safely and effectively. See full prescribing information for MIEBO.
MIEBO ®(perfluorohexyloctane ophthalmic solution), for topical ophthalmic use
Initial U.S. Approval: 2023RECENT MAJOR CHANGES
Contraindications, Hypersensitivity ( 4.1) 10/2025
INDICATIONS AND USAGE
MIEBO (perfluorohexyloctane ophthalmic solution) is a semifluorinated alkane indicated for treatment of the signs and symptoms of dry eye disease. ( 1)
DOSAGE AND ADMINISTRATION
Instill one drop of MIEBO four times daily into each eye. ( 2.1)
DOSAGE FORMS AND STRENGTHS
Ophthalmic solution: 100% perfluorohexyloctane. ( 3)
CONTRAINDICATIONS
Hypersensitivity. ( 4.1)
ADVERSE REACTIONS
Most common ocular adverse reaction was blurred vision. Blurred vision was reported in less than 4% of individuals. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2025
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Administration Instructions
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
4.1 Hypersensitivity
5 WARNINGS AND PRECAUTIONS
5.1 Use with Contact Lenses
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
Instill one drop of MIEBO four times daily into affected eye(s).
Contact lenses should be removed prior to and for at least 30 minutes after the administration of MIEBO.
2.2 Administration Instructions
Step 1.Remove the cap from eye drop bottle.
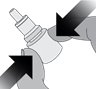 Step 2.Holding the bottle upright, gently squeeze the bottle.
Step 2.Holding the bottle upright, gently squeeze the bottle.
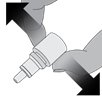 Step 3.While squeezing, turn the bottle upside down and release the pressure (drawing air into the bottle).
Step 3.While squeezing, turn the bottle upside down and release the pressure (drawing air into the bottle).
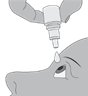 Step 4.Keeping the bottle upside down, place the bottle above your eye and squeeze it again to release a drop into your eye.
Step 4.Keeping the bottle upside down, place the bottle above your eye and squeeze it again to release a drop into your eye.
Repeat steps 1 - 4 for the second affected eye.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
4.1 Hypersensitivity
MIEBO is contraindicated in patients with a history of hypersensitivity reaction to perfluorohexyloctane [see Adverse Reactions ( 6.1)] .
- 5 WARNINGS AND PRECAUTIONS
-
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In patients with DED, 614 patients received at least one dose of MIEBO in two randomized controlled clinical trials across 68 sites in the United States. The most common ocular adverse reaction was blurred vision. Blurred vision and conjunctival redness were reported in 1-3% of individuals.
In four premarketing studies (three open-label [n=127], one randomized [n=24 treated with at least one dose of perfluorohexyloctane]) the most common adverse reaction was hypersensitivity.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no adequate and well controlled studies with MIEBO in pregnant women.
In animal reproduction studies with oral administration of perfluorohexyloctane during the period of organogenesis, no adverse maternal or developmental effects were observed in rats at doses up to 162 times the recommended human ophthalmic dose (RHOD) ( see Data). Maternal toxicity, miscarriages and reduced fetal weights were observed in rabbits at all doses tested, with the lowest dose as 41 times the RHOD.
All pregnancies have a risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects is 2 to 4%, and of miscarriage is 15 to 20%, of clinically recognized pregnancies.
Data
Animal Data
An embryofetal study was conducted in pregnant rabbits administered perfluorohexyloctane by oral gavage on gestation days 6 to 19, to target the period of organogenesis. Perfluorohexyloctane produced maternal toxicity, characterized by reduced body weight gain and food consumption, and miscarriages at all doses tested, with the lowest dose as ≥ 250 mg/kg/day (41 times the RHOD based on body surface area). Reduced fetal weights were also observed at ≥ 250 mg/kg/day but no fetal mortality or malformations. A no observed adverse effect level (NOAEL) for maternal toxicity was not established in rabbits.
An embryofetal study was conducted in pregnant rats administered perfluorohexyloctane by oral gavage on gestation days 6 to 17, to target the period of organogenesis. There was no evidence of embryofetal toxicity or teratogenicity at doses up to 2,000 mg/kg/day (162 times the RHOD).
8.2 Lactation
There are no data on the presence of perfluorohexyloctane in human milk, the effects on the breastfed infant, or the effects on milk production. The lack of clinical data during lactation precludes a clear determination of the risk of MIEBO to an infant during lactation; however, the developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for MIEBO.
-
11 DESCRIPTION
MIEBO ®(perfluorohexyloctane ophthalmic solution) is a sterile, clear and colorless liquid containing 100% perfluorohexyloctane, for topical ophthalmic use.
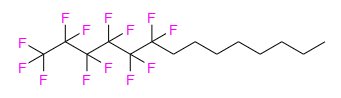
The active ingredient is 1,1,1,2,2,3,3,4,4,5,5,6,6-tridecafluorotetradecane and is a semifluorinated alkane. It has a molecular formula of C 14H 17F 13and a molecular weight of 432.26 g/mol. The chemical structure is:
Perfluorohexyloctane is practically immiscible with water. It is miscible with ethanol and most
organic solvents. Each multiple-dose bottle contains 3 mL of perfluorohexyloctane, 1.338 g/mL as a clear and colorless liquid.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Perfluorohexyloctane, a semifluorinated alkane, contains 6 perfluorinated carbon atoms and 8 hydrogenated carbon atoms. Perfluorohexyloctane forms a monolayer at the air-liquid interface of the tear film which can be expected to reduce evaporation. The exact mechanism of action for MIEBO in DED is not known.
12.3 Pharmacokinetics
The pharmacokinetics of perfluorohexyloctane following topical ocular administration of MIEBO has not been quantitatively characterized in humans. A single pharmacokinetic (PK) study was conducted that showed low systemic perfluorohexyloctane blood levels after topical ocular administration. Perfluorohexyloctane was not metabolized by human liver microsomes in vitro.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals have not been conducted to evaluate the carcinogenic potential of perfluorohexyloctane.
Perfluorohexyloctane was not mutagenic or clastogenic in a standard battery of genotoxicity tests, including a bacterial mutagenicity assay (Ames assay), an in vitro chromosome aberration assay using human peripheral lymphocytes, and an in vivo bone marrow micronucleus assay in rats.
-
14 CLINICAL STUDIES
In two randomized, multicenter, double-masked, saline-controlled trials (GOBI and MOJAVE), a total of 1,217 patients with a history of DED and clinical signs of meibomian gland dysfunction were randomized to MIEBO or saline 0.6% (1:1 ratio) to evaluate safety and efficacy after receiving MIEBO four times daily (QID) for 57 days.The mean age of the 614 patients who received MIEBO was 57 years (range, 19-87 years). The majority of patients were female (76%).
Effects on Signs of Dry Eye Disease
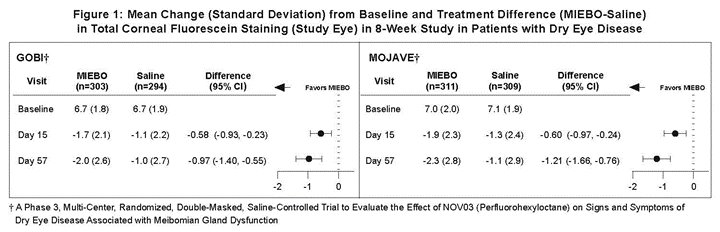
Total corneal fluorescein staining (tCFS) was recorded at each study visit using a standardized grading system of 0-3 for each of the five areas on the cornea (inferior, superior, central, nasal, and temporal), totaling a maximum tCFS score for each eye of 15. The average baseline tCFS was approximately 6.7 in GOBI and 7.0 in MOJAVE. At Days 15 and 57, a statistically significant reduction in tCFS favoring MIEBO was observed in both studies (Figure 1).
Effects on Symptoms of Dry Eye Disease
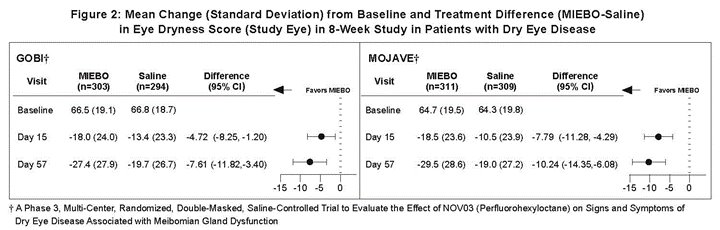
Eye dryness score was rated by patients using a visual analogue scale (VAS) (0=no discomfort, 100=maximal discomfort) at each study visit. The baseline VAS eye dryness average score was approximately 67 in GOBI and 65 in MOJAVE. At Days 15 and 57, a statistically significant reduction in VAS eye dryness score favoring MIEBO was observed in both studies (Figure 2).
-
16 HOW SUPPLIED/STORAGE AND HANDLING
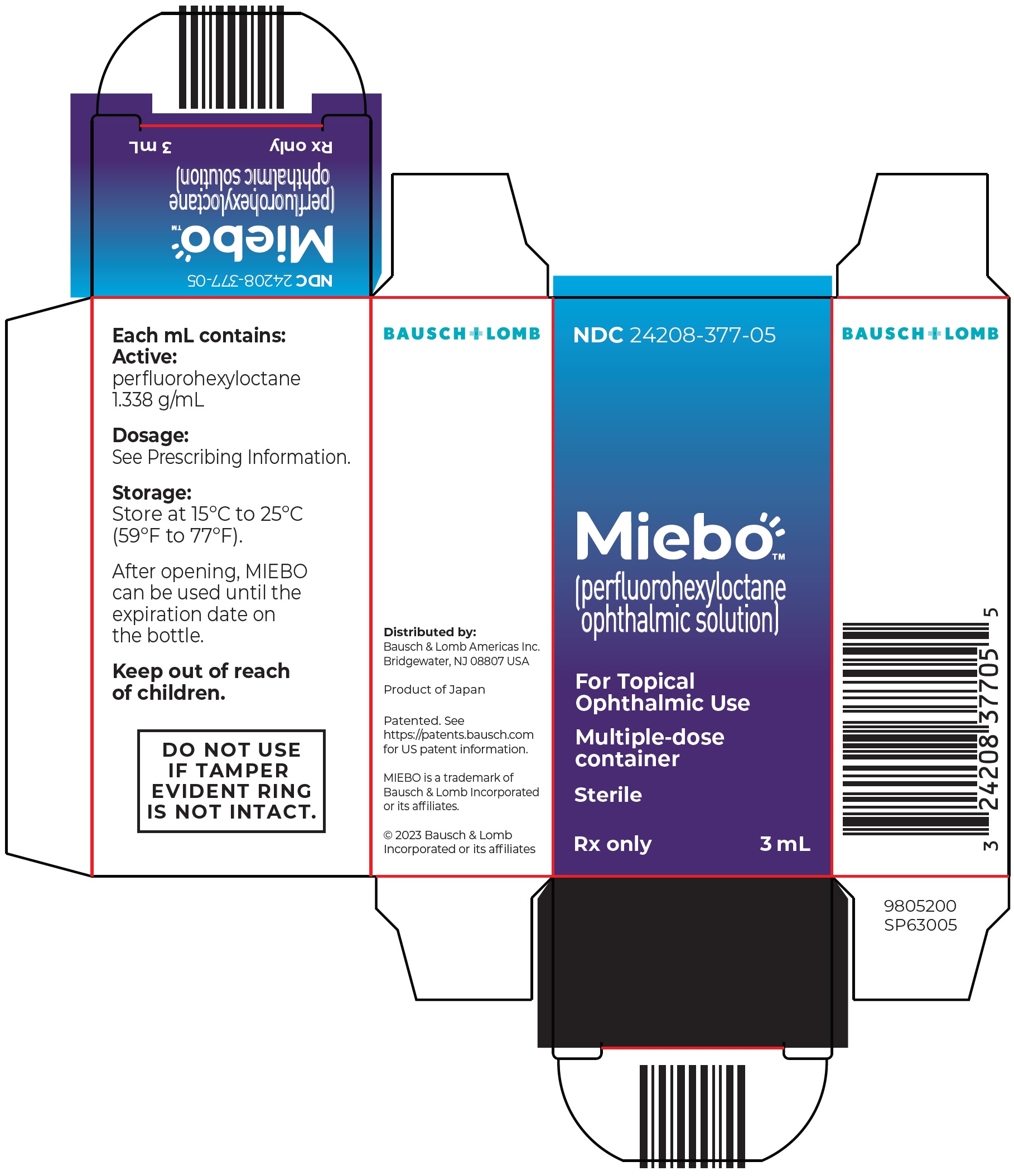
MIEBO ®(perfluorohexyloctane ophthalmic solution) is supplied as a sterile, clear and colorless liquid in multiple-dose 5 mL polypropylene bottles with dropper tips and screw caps, packaged in a carton - NDC: 24208-377-05.
Storage
Store MIEBO at 15ºC to 25ºC (59ºF to 77ºF). After opening, MIEBO can be used until the expiration date on the bottle.
-
17 PATIENT COUNSELING INFORMATION
Use with Contact Lenses
Advise patients that contact lenses should be removed prior to and for at least 30 minutes after administration of MIEBO.
Administration Instructions
Advise patients to instill one drop of MIEBO four times daily into each eye as depicted in the Administration Instructions [see Dosage and Administration ( 2.2)] .
Distributed by:
Bausch & Lomb Americas Inc.
Bridgewater, NJ 08807 USA
Patented. See https://patents.bausch.comfor US patent information.
MIEBO is a trademark of Bausch & Lomb Incorporated or its affiliates.
© 2025 Bausch & Lomb Incorporated or its affiliates
9805301
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MIEBO
perfluorohexyloctane solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 24208-377 Route of Administration OPHTHALMIC Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PERFLUOROHEXYLOCTANE (UNII: 7VYX4ELWQM) (PERFLUOROHEXYLOCTANE - UNII:7VYX4ELWQM) PERFLUOROHEXYLOCTANE 1 mg in 1 mg Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 24208-377-05 1 in 1 CARTON 05/18/2023 1 3 mg in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 24208-377-01 1 in 1 CARTON 05/18/2023 2 3 mg in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA216675 05/18/2023 Labeler - Bausch & Lomb Incorporated (196603781) Establishment Name Address ID/FEI Business Operations Alliance Medical Products, Inc. (dba Siegfried Irvine) 102688657 manufacture(24208-377)
Trademark Results [MIEBO]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 MIEBO 97424093 not registered Live/Pending |
Bausch + Lomb Ireland Limited 2022-05-23 |
 MIEBO 90360371 not registered Live/Pending |
Bausch + Lomb Ireland Limited 2020-12-04 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.