MICONAZOLE NITRATE cream
Miconazole Nitrate by
Drug Labeling and Warnings
Miconazole Nitrate by is a Otc medication manufactured, distributed, or labeled by Taro Pharmaceuticals U.S.A., Inc., Taro Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
For vaginal use only
Ask a doctor before use if you have
- vaginal itching and discomfort for the first time
- lower abdominal, back or shoulder pain, fever, chills, nausea, vomiting, or foul-smelling vaginal discharge. You may have a more serious condition.
- vaginal yeast infections often (such as once a month or 3 in 6 months). You could be pregnant or have a serious underlying medical cause for your symptoms, including diabetes or a weakened immune system.
- been exposed to the human immunodeficiency virus (HIV) that causes AIDS
Ask a doctor or pharmacist before use if you are taking the prescription blood thinning medicine warfarin, because bleeding or bruising may occur
When using this product
- do not use tampons, douches, spermicides, or other vaginal products. Condoms and diaphragms may be damaged and fail to prevent pregnancy or sexually transmitted diseases (STDs).
- do not have vaginal intercourse
- mild increase in vaginal burning, itching or irritation may occur
- if you do not get complete relief ask a doctor before using another product
-
Directions
- before using this product read the enclosed consumer information leaflet for complete directions and information
- adults and children 12 years of age and over:
- applicator: insert 1 applicatorful into the vagina at bedtime for 7 nights in a row. Wash applicator after use.
- use the same tube of cream if you have itching and irritation on the skin outside the vagina. Squeeze a small amount of cream onto your fingertip. Apply to itchy, irritated skin outside the vagina (vulva). Use 2 times daily for up to 7 days as needed.
- children under 12 years of age: ask a doctor
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
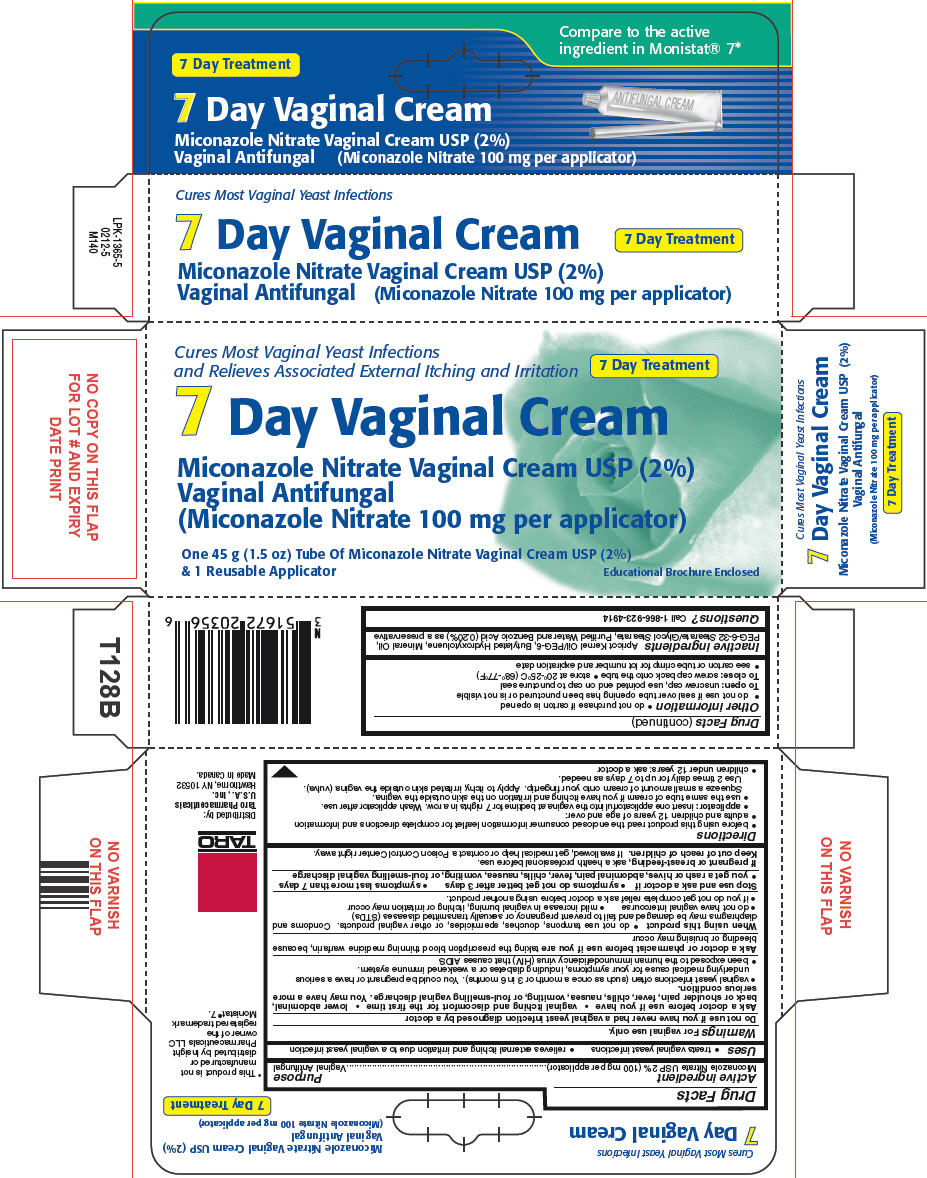
PRINCIPAL DISPLAY PANEL - 45 g Tube Carton
CURES most vaginal YEAST INFECTIONS
Relieves associated external itching and irritationNDC: 51672-2035-6
7 Day Treatment
7 Day Vaginal Cream
Miconazole Nitrate Vaginal Cream USP (2%)
(Miconazole Nitrate 100 mg per application)
Vaginal AntifungalOne 45 g (1.5 oz) tube of Miconazole Nitrate Vaginal Cream USP (2%)
& 1 Reusable ApplicatorConsumer Information Leaflet Enclosed
-
INGREDIENTS AND APPEARANCE
MICONAZOLE NITRATE
miconazole nitrate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 51672-2035 Route of Administration VAGINAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Miconazole Nitrate (UNII: VW4H1CYW1K) (Miconazole - UNII:7NNO0D7S5M) Miconazole Nitrate 20 mg in 1 g Inactive Ingredients Ingredient Name Strength Butylated Hydroxytoluene (UNII: 1P9D0Z171K) Mineral Oil (UNII: T5L8T28FGP) Water (UNII: 059QF0KO0R) Benzoic Acid (UNII: 8SKN0B0MIM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 51672-2035-6 1 in 1 CARTON 01/13/1997 1 45 g in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product 2 NDC: 51672-2035-7 1 in 1 CARTON 01/13/1997 2 45 g in 1 TUBE, WITH APPLICATOR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074444 01/13/1997 Labeler - Taro Pharmaceuticals U.S.A., Inc. (145186370) Establishment Name Address ID/FEI Business Operations Taro Pharmaceuticals Inc. 206263295 MANUFACTURE(51672-2035)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.