BabyGanics™ Alcohol-Free Foaming Hand Sanitizer Mandarin
BabyGanics Alcohol-Free Foaming Hand Sanitizer Mandarin by
Drug Labeling and Warnings
BabyGanics Alcohol-Free Foaming Hand Sanitizer Mandarin by is a Otc medication manufactured, distributed, or labeled by KAS Direct LLC dba BabyGanics. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BABYGANICS ALCOHOL-FREE FOAMING HAND SANITIZER MANDARIN- benzalkonium chloride liquid
KAS Direct LLC dba BabyGanics
----------
BabyGanics™ Alcohol-Free Foaming Hand Sanitizer Mandarin
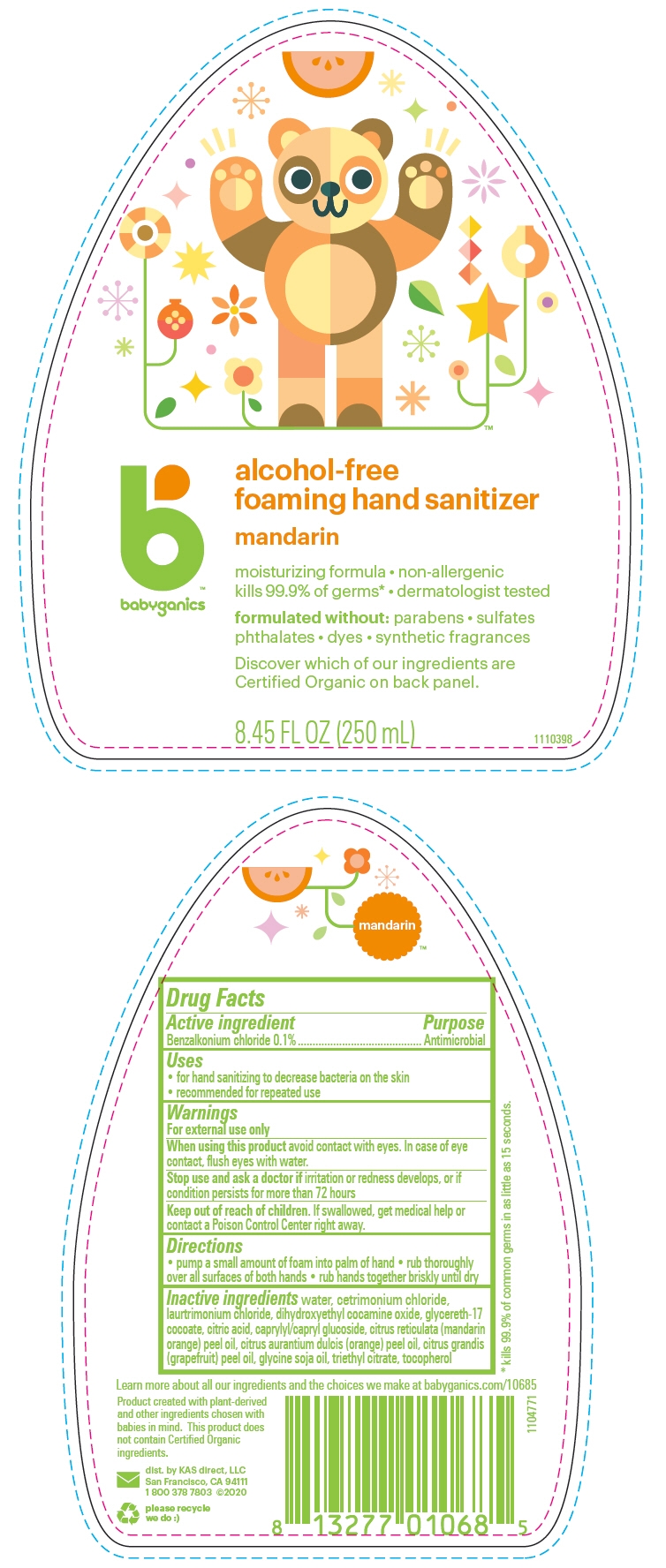
Warnings
For external use only
Directions
- pump a small amount of foam into palm of hand
- rub thoroughly over all surfaces of both hands
- rub hands together briskly until dry
Inactive ingredients
water, cetrimonium chloride, laurtrimonium chloride, dihydroxyethyl cocamine oxide, glycereth-17 cocoate, citric acid, caprylyl/capryl glucoside, citrus reticulata (mandarin orange) peel oil, citrus aurantium dulcis (orange) peel oil, citrus grandis (grapefruit) peel oil, glycine soja oil, triethyl citrate, tocopherol
PRINCIPAL DISPLAY PANEL - 250 mL Bottle Label
babyganics™
alcohol-free
foaming hand sanitizer
mandarin
moisturizing formula non-allergenic
kills 99.9% of germs* dermatologist tested
formulated without: parabens sulfates
phthalates dyes synthetic fragrances
Discover which of our ingredients are
Certified Organic on back panel.
8.45 FL OZ (250 mL)
1110398
| BABYGANICS ALCOHOL-FREE FOAMING HAND SANITIZER MANDARIN
benzalkonium chloride liquid |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| Labeler - KAS Direct LLC dba BabyGanics (002764605) |