QBREXZA- glycopyrronium cloth
Qbrexza by
Drug Labeling and Warnings
Qbrexza by is a Prescription medication manufactured, distributed, or labeled by Journey Medical Corporation, Aphena Pharma Solutions, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use QBREXZA™ safely and effectively. See full prescribing information for QBREXZA.
QBREXZA (glycopyrronium) cloth, 2.4%, for topical use
Initial U.S. Approval: 2018INDICATIONS AND USAGE
Qbrexza is an anticholinergic indicated for topical treatment of primary axillary hyperhidrosis in adults and pediatric patients 9 years of age and older (1).
DOSAGE AND ADMINISTRATION
For topical use only. Apply Qbrexza once daily to both axillae using a single cloth (2).
DOSAGE FORMS AND STRENGTHS
Cloth: A single-use cloth pre-moistened with 2.4% glycopyrronium solution (3).
CONTRAINDICATIONS
Qbrexza is contraindicated in patients with medical conditions that can be exacerbated by the anticholinergic effect of Qbrexza (e.g., glaucoma, paralytic ileus, unstable cardiovascular status in acute hemorrhage, severe ulcerative colitis, toxic megacolon complicating ulcerative colitis, myasthenia gravis, Sjogren’s syndrome) (4)
WARNINGS AND PRECAUTIONS
- Worsening of urinary retention: Use with caution in patients with a history or presence of documented urinary retention (5.1).
- Control of body temperature: In the presence of high ambient temperature, heat illness may occur; avoid use if patients develop generalized lack of sweating when exposed to hot or very warm environmental temperatures (5.2).
- Operating machinery or an automobile: Transient blurred vision may occur with use of Qbrexza. If blurred vision occurs, discontinue use of Qbrexza until symptoms resolve; avoid operating a motor vehicle or other machinery until symptoms resolve (5.3).
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥2%) are dry mouth, mydriasis, oropharyngeal pain, headache, urinary hesitation, vision blurred, nasal dryness, dry throat, dry eye, dry skin, constipation. Local skin reactions, including erythema, burning/stinging and pruritus were also common (>5%) (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Dermira at 1-877-337-5553 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
Coadministration of Qbrexza with anticholinergic medications may result in additive interaction leading to an increase in anticholinergic adverse effects. Avoid coadministration of Qbrexza with other anticholinergic-containing drugs (7)
USE IN SPECIFIC POPULATIONS
- Pediatric use: Safety and efficacy are not established in patients under 9 years of age (8.4).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Worsening of Urinary Retention
5.2 Control of Body Temperature
5.3 Operating Machinery or an Automobile
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Anticholinergics
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Efficacy and Safety Trials
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
For topical use only.
Qbrexza is for topical use in the underarm area only and not for use in other body areas.
Qbrexza is administered by a single-use pre-moistened cloth packaged in individual pouches. Qbrexza should be applied to clean dry skin on the underarm areas only. Qbrexza should not be used more frequently than once every 24 hours.
Tear open the pouch and pull out the cloth, unfold the cloth, and wipe it across one entire underarm once. Using the same cloth, wipe the other underarm once. A single cloth should be used to apply Qbrexza to both underarms.
Wash hands immediately with soap and water after applying and discarding the Qbrexza cloth. Qbrexza may cause temporary dilation of the pupils and blurred vision if it comes in contact with the eyes. Avoid transfer of Qbrexza to the periocular area [see Warnings and Precautions (5.3)].
Do not apply Qbrexza to broken skin. Avoid using Qbrexza with occlusive dressings.
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Qbrexza is contraindicated in patients with medical conditions that can be exacerbated by the anticholinergic effect of Qbrexza (e.g., glaucoma, paralytic ileus, unstable cardiovascular status in acute hemorrhage, severe ulcerative colitis, toxic megacolon complicating ulcerative colitis, myasthenia gravis, Sjogren’s syndrome).
-
5 WARNINGS AND PRECAUTIONS
5.1 Worsening of Urinary Retention
Qbrexza should be used with caution in patients with a history or presence of documented urinary retention. Prescribers and patients should be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, distended bladder), especially in patients with prostatic hypertrophy or bladder-neck obstruction. Instruct patients to discontinue use immediately and consult a physician should any of these signs or symptoms develop.
Patients with a history of urinary retention were not included in the clinical studies.
5.2 Control of Body Temperature
In the presence of high ambient temperature, heat illness (hyperpyrexia and heat stroke due to decreased sweating) can occur with the use of anticholinergic drugs such as Qbrexza. Advise patients using Qbrexza to watch for generalized lack of sweating when in hot or very warm environmental temperatures and to avoid use if not sweating under these conditions.
5.3 Operating Machinery or an Automobile
Transient blurred vision may occur with use of Qbrexza. If blurred vision occurs, the patient should discontinue use until symptoms resolve. Patients should be warned not to engage in activities that require clear vision such as operating a motor vehicle or other machinery, or performing hazardous work until the symptoms have resolved.
-
6 ADVERSE REACTIONS
The following adverse reactions are described in greater detail in other sections
- Worsening of Urinary Retention [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In two double-blind, vehicle-controlled clinical trials (Trial 1 [NCT02530281] and Trial 2 [NCT02530294]) of 459 subjects treated with Qbrexza once daily and 232 treated with vehicle, subjects were 9 to 76 years of age, 47% male, and the percentages of White, Black (including African Americans), and Asian subjects were 82%, 12%, and 1%, respectively.
Table 1 summarizes the most frequent adverse reactions (≥2%) in subjects with primary axillary hyperhidrosis treated with Qbrexza.
Table 1: Adverse Reactions Occurring in ≥2% of Subjects Adverse Reactions Qbrexza
(N=459)
n (%)Vehicle
(N=232)
n (%)Dry mouth 111 (24.2%) 13 (5.6%) Mydriasis 31 (6.8%) 0 Oropharyngeal pain 26 (5.7%) 3 (1.3%) Headache 23 (5.0%) 5 (2.2%) Urinary hesitation 16 (3.5%) 0 Vision blurred 16 (3.5%) 0 Nasal dryness 12 (2.6%) 1 (0.4%) Dry throat 12 (2.6%) 0 Dry eye 11 (2.4%) 1 (0.4%) Dry skin 10 (2.2%) 0 Constipation 9 (2.0%) 0 Table 2 shows the most frequently reported local skin reactions, which were relatively common in both the Qbrexza and vehicle groups.
Table 2: Local Skin Reactions a Patients with a post-baseline local skin reaction assessment Local Skin Reactions Qbrexza
(N=454)a
n (%)Vehicle
(N=231)a
n (%)Erythema 77 (17.0%) 39 (16.9%) Burning/stinging 64 (14.1%) 39 (16.9%) Pruritus 37 (8.1%) 14 (6.1%) In an open-label safety trial (NCT02553798), 564 subjects were treated for up to an additional 44 weeks after completing Trial 1 or Trial 2. Adverse reactions occurring at a frequency ≥2.0% were: dry mouth (16.9%), vision blurred (6.7%), nasopharyngitis (5.8%), mydriasis (5.3%), urinary hesitation (4.2%), nasal dryness (3.6%), dry eye (2.9%), pharyngitis (2.2%), and application site reactions (pain [6.4%], dermatitis [3.8%], pruritus [3.8%], rash [3.8%], erythema [2.4%]).
-
7 DRUG INTERACTIONS
7.1 Anticholinergics
Coadministration of Qbrexza with anticholinergic medications may result in additive interaction leading to an increase in anticholinergic adverse effects [see Warnings and Precautions (5) and Adverse Reactions (6)]. Avoid coadministration of Qbrexza with other anticholinergic-containing drugs.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data on Qbrexza use in pregnant women to inform a drug-associated risk for adverse developmental outcomes. In pregnant rats, daily oral administration of glycopyrrolate (glycopyrronium bromide) during organogenesis did not result in an increased incidence of gross external or visceral defects [see Data]. When glycopyrrolate was administered intravenously to pregnant rabbits during organogenesis, no adverse effects on embryo-fetal development were seen. The available data do not support relevant comparisons of systemic glycopyrronium exposures achieved in the animal studies to exposures observed in humans after topical use of Qbrexza.
The estimated background risks of major birth defects and miscarriage for the indicated population are unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Animal Data
Glycopyrrolate was orally administered to pregnant rats at dosages of 50, 200, and 400 mg/kg/day during the period of organogenesis. Glycopyrrolate had no effect on maternal survival, but significantly reduced mean maternal body weight gain over the period of dosing at all dosages evaluated. Mean fetal weight was significantly reduced in the 200 and 400 mg/kg/day dose groups. There were two litters with all resorbed fetuses in the 400 mg/kg/day dose group. There were no effects of treatment on the incidence of gross external or visceral defects. Minor treatment-related skeletal effects included reduced ossification of various bones in the 200 and 400 mg/kg/day dose groups; these skeletal effects were likely secondary to maternal toxicity.
Glycopyrrolate was intravenously administered to pregnant rabbits at dosages of 0.1, 0.5, and 1.0 mg/kg/day during the period of organogenesis. Glycopyrrolate did not affect maternal survival under the conditions of this study. Mean maternal body weight gain and mean food consumption over the period of dosing were lower than the corresponding control value in the 0.5 and 1.0 mg/kg/day treatment groups. There were no effects of treatment on fetal parameters, including fetal survival, mean fetal weight, and the incidence of external, visceral, or skeletal defects.
Female rats that were pregnant or nursing were orally dosed with glycopyrrolate daily at dosages of 0, 50, 200, or 400 mg/kg/day, beginning on day 7 of gestation, and continuing until day 20 of lactation. Mean body weight of pups in all treatment groups was reduced compared to the control group during the period of nursing, but eventually recovered to be comparable to the control group, post-weaning. No other notable delivery or litter parameters were affected by treatment in any group, including no effects on mean duration of gestation or mean numbers of live pups per litter. No treatment-related effects on survival or adverse clinical signs were observed in pups. There were no effects of maternal treatment on behavior, learning, memory, or reproductive function of pups.
8.2 Lactation
Risk Summary
There are no data on the presence of glycopyrrolate or its metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Qbrexza and any potential adverse effects on the breastfed infant from Qbrexza or from the underlying maternal condition.
8.4 Pediatric Use
The safety, effectiveness and pharmacokinetics of Qbrexza have been established in pediatric patients age 9 years and older for topical treatment of primary axillary hyperhidrosis [see Clinical Pharmacology (12.3)]. Use of Qbrexza in this age group is supported by evidence from two multicenter, randomized, double-blind, parallel-group, vehicle-controlled 4-week trials which included 34 pediatric subjects 9 years and older [see Adverse Reactions (6.1) and Clinical Studies (14)]. The safety and effectiveness of Qbrexza have not been established in pediatric patients under 9 years of age.
8.5 Geriatric Use
Clinical trials of Qbrexza did not include sufficient numbers of subjects age 65 years and older to determine whether they respond differently from younger subjects.
8.6 Renal Impairment
The elimination of glycopyrronium is severely impaired in patients with renal failure [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Because glycopyrronium is a quaternary amine which does not easily cross the blood-brain barrier, symptoms of glycopyrronium overdosage are generally more peripheral in nature rather than central compared to other anticholinergic agents. Associated signs and symptoms related to excessive anticholinergic activity may include flushing, hyperthermia, tachycardia, ileus, urinary retention, loss of ocular accommodation and light sensitivity due to mydriasis.
In the case of overdose when symptoms are severe or life threatening, therapy may include:
- Managing per standard of care any acute conditions such as hyperthermia, coma, and/or seizures, as applicable, and managing any myoclonic or choreoathetoid movements which may lead to rhabdomyolysis in some cases of anticholinergic overdosage
- Managing severe urinary retention with catheterization if not spontaneously reversed within several hours
- Providing cardiovascular support and/or controlling arrhythmias
- Maintaining an open airway, providing ventilation as necessary
- Administering a quaternary ammonium anticholinesterase such as neostigmine to help alleviate severe and/or life threatening peripheral anticholinergic effects.
Topical overdosing of Qbrexza could result in an increased incidence or severity of local skin reactions. Administration of Qbrexza under occlusive conditions may result in an increase in anticholinergic effects, including dry mouth and urinary hesitation.
-
11 DESCRIPTION
Qbrexza (glycopyrronium) cloth, 2.4% is an anticholinergic drug available as a clear, colorless to pale yellow solution on a single-use pre-moistened cloth (an absorbent polypropylene pad) packaged in a pouch for topical administration. Each pouch contains 105 mg glycopyrronium tosylate, equivalent to 66 mg of glycopyrronium. The inactive ingredients are citric acid, dehydrated alcohol, purified water, and sodium citrate.
Glycopyrronium tosylate is chemically described as pyrrolidinium, 3-[(2-cyclopentyl-2-hydroxy-2-phenylacetyl)oxy]-1,1-dimethyl-, 4-methylbenzensulfonate, hydrate (1:1:1) with an empirical formula of C26H37NO7S and a molecular weight of 507.6. The structural formula is represented below:
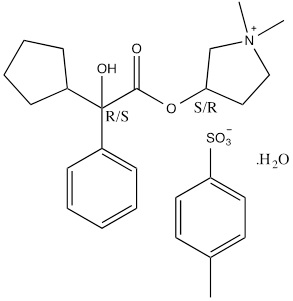
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Glycopyrronium is a competitive inhibitor of acetylcholine receptors that are located on certain peripheral tissues, including sweat glands. In hyperhidrosis, glycopyrronium inhibits the action of acetylcholine on sweat glands, reducing sweating.
12.3 Pharmacokinetics
Absorption
The pharmacokinetics of glycopyrronium were evaluated in adult and pediatric patients with primary axillary hyperhidrosis following Qbrexza once daily applied to the axillae for 5 days. The mean ± SD exposures of glycopyrronium are presented in Tables 3 and 4. There was no evidence of accumulation.
Table 3: Mean ± SD Plasma Exposures of Glycopyrronium in Adults Following Qbrexza Once Daily for 5 days Abbreviations: Maximum concentration (Cmax), Area under the time concentration curve
(AUC) between 0 and 6 hours following administration of Qbrexza (AU0-6h), AUC between
0 and 24 hours following administration of Qbrexza (AUC0-24h)Parameter Adult Patients Cmax (ng/mL) 0.08 ± 0.04 AUC0-6h (h∗ng/mL) 0.2 ± 0.14 AUC0-24h (h∗ng/mL) 0.88 ± 0.57 Median Tmax (Range) (h) 1 (0, 10) Distribution
After IV administration, glycopyrronium has a mean volume of distribution in children aged 1 to 14 years of approximately 1.3 to 1.8 L/kg, with a range from 0.7 to 3.9 L/kg. In adults aged 60-75 years, the volume of distribution was lower (0.42 L/kg ± 0.22).
Elimination
Metabolism
A small proportion of glycopyrronium is metabolized following IV administration. The metabolic pathway for glycopyrronium is not characterized.
Excretion
Following administration of a single radiolabeled IV glycopyrronium dose to adult subjects who underwent surgery for cholelithiasis, approximately 85% of total radioactivity was excreted in urine and < 5% was present in bile drainage. Greater than 80% of the radioactivity in both urine and bile was unchanged drug.
Specific Populations
The pharmacokinetics of glycopyrronium were not evaluated in pregnant women or patients with hepatic impairment.
Pediatric Subjects
The mean ± SD exposures of glycopyrronium in pediatric subjects following Qbrexza once daily for 5 days are presented in Table 4. There was no evidence of accumulation.
Table 4: Mean ± SD Plasma Exposures of Glycopyrronium in Pediatric Subjects Aged 10 to 17 years Following Qbrexza Once Daily for 5 days Parameter Pediatric Patients Cmax (ng/mL) 0.07 ± 0.06 AUC0-6h (h∗ng/mL) 0.18 ± 0.13 AUC0-24h (h∗ng/mL) Not calculated Median Tmax (Range) (h) 1.5 (0, 6) Patients with Renal Impairment
Following a 4 mcg/kg IV dose of a glycopyrronium formulation for IV use, mean glycopyrronium AUC (10.6 mcg·h/L), CL (0.43 L/h/kg) and 3-hour urinary excretion (0.7%) were significantly different in uremic subjects undergoing renal transplantation surgery than those of healthy subjects (3.73 mcg·h/L, 1.14 L/h/kg, and 50%, respectively).
Pharmacokinetics of Qbrexza in subjects with renal impairment has not been studied.
In Vitro Studies
In vitro studies indicated that under the conditions of clinical use, Qbrexza is not expected to induce cytochrome P450 (CYP) enzymes 1A2, 2B6 and 3A4; or inhibit 1A2, 2B6, 2C8, 2C9, 2C19, 2D6 and 3A4.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Glycopyrronium tosylate was not carcinogenic when topically applied to rats daily for up to 24 months in solution at concentrations of 1%, 2%, and 4% w/w.
When glycopyrrolate was administered via oral gavage to mice for up to 24 months at dosages of 2.5, 7, and 20 mg/kg/day in both genders, no significant changes in tumor incidence were observed when compared to control.
When glycopyrrolate was administered via oral gavage to rats for up to 24 months at dosages of 5, 15, and 40 mg/kg/day in both genders, no significant changes in tumor incidence were observed when compared to control.
Glycopyrrolate was negative in a battery of genetic toxicology studies that included a bacterial reverse mutation (Ames) assay, a mouse lymphoma assay conducted with L5178Y/TK+/- cells, and an in vivo micronucleus assay with mice. Glycopyrronium tosylate was negative in an Ames assay.
Glycopyrrolate was assessed for effects on fertility or general reproductive function in rats. Rats of both genders received glycopyrrolate at dosages up to 100 mg/kg/day via oral gavage. No treatment-related effects on fertility or reproductive parameters were observed in either gender.
-
14 CLINICAL STUDIES
14.1 Efficacy and Safety Trials
Two randomized, vehicle-controlled multicenter trials, Trial 1 (NCT02530281) and Trial 2 (NCT02530294), were conducted in subjects with primary axillary hyperhidrosis and enrolled a total of 697 subjects 9 years of age or older. Inclusion criteria required that prior to the start of treatment, all subjects produce at least 50 mg of sweat in each axilla over a 5-minute period and rate the severity of their sweating daily over a week with a mean score of 4 or higher on the ASDD item #2, a patient reported outcome instrument scored from 0 (no sweating) to 10 (worst possible sweating). The median sweat production over 5 minutes at baseline was 122 mg in the Qbrexza arm and 113 mg in the vehicle arm in Trial 1, and 127 mg in the Qbrexza arm and 117 mg in the vehicle arm in Trial 2. The average weekly mean score on the ASDD item #2 at baseline was approximately 7.2 across both trials.
Subjects were randomized to receive either Qbrexza or vehicle applied once daily to each axilla. The co-primary endpoints were the proportion of subjects having at least a 4-point improvement from baseline in the weekly mean ASDD item #2 score at Week 4 and the mean absolute change from baseline in gravimetrically measured sweat production at Week 4.
Clinical Response
The results of Trial 1 and Trial 2 are presented in Table 5 below.
Table 5: Primary Efficacy Outcomes in Subjects with Primary Axillary Hyperhidrosis Trial 1 Trial 2 Qbrexza, 2.4%
N = 229Vehicle
N = 115Qbrexza
2.4%
N = 234Vehicle
N = 119ASDD Item #2 Response at Week 4: Proportion of subjects with at least a 4-point
improvement from baseline in the weekly
mean ASDD item #2 at Week 453% 28% 66% 27% Change from Baseline in Sweat Production at Week 4 (mg/5 minutes): Median -81 -66 -79 -58 25th percentile, 75th percentile -149, -40 -106, -28 -144, -45 -122, -21 -
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Qbrexza is supplied as:
A single-use cloth pre-moistened with a 2.4% glycopyrronium solution in a pouch
Carton of 30 pouches NDC: 70428-011-12
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Worsening of Urinary Retention
Instruct patients to be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, distended bladder). Instruct patients to discontinue use and consult a physician immediately should any of these signs or symptoms develop.
Control of Body Temperature (Risk of Overheating or Heat Illness)
In the presence of high ambient temperature, heat illness due to decreased sweating can occur with the use of anticholinergic drugs such as Qbrexza. Advise patients using Qbrexza to watch for generalized lack of sweating when in hot or very warm environmental temperatures and to avoid use if not sweating under these conditions.
Operating Machinery or an Automobile
Transient blurred vision may occur with Qbrexza. If this occurs, instruct patients to contact their healthcare provider, discontinue use of Qbrexza and avoid operating a motor vehicle or other machinery, or performing hazardous work until symptoms resolve.
Instructions for Administering Qbrexza
It is important for patients to understand how to correctly apply Qbrexza (see Patient Information).
- Instruct patients to use one cloth to apply Qbrexza to both axillae by wiping the cloth across one underarm, ONE TIME.
- Using the same cloth, apply the medication to the other underarm, ONE TIME.
- Inform patients that Qbrexza can cause temporary dilation of the pupils and blurred vision if it comes in contact with the eyes.
- Instruct patients to wash their hands with soap and water immediately after discarding the used cloth.
- Remind patients not to apply Qbrexza to other body areas or to broken skin. Instruct patients to avoid using Qbrexza with occlusive dressings.
- Qbrexza is flammable; avoid use near heat or flame.
Manufactured for:
Dermira, Inc.
Menlo Park, CA 94025Version 1, June 2018
-
PATIENT PACKAGE INSERT
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 06/2018 PATIENT INFORMATION
Qbrexza™ (kew brex’ zah)
(glycopyrronium)
cloth, 2.4%Important Information: Qbrexza is for use on the skin in the underarm area only.
What is Qbrexza?
Qbrexza is a prescription anticholinergic medicine used on the skin (topical) to treat excessive underarm sweating (primary axillary hyperhidrosis) in adults and children 9 years of age and older.
It is not known if Qbrexza is safe and effective in children under 9 years of age.
Who should not use Qbrexza?
Do not use Qbrexza if you have certain medical conditions that can be made worse by taking an anticholinergic medicine such as glaucoma, severe ulcerative colitis or certain other serious bowel problems associated with severe ulcerative colitis, myasthenia gravis, and Sjogren’s syndrome.
Talk to your healthcare provider if you are not sure if you have a medical condition that can be made worse by taking an anticholinergic medicine.
Before using Qbrexza, tell your healthcare provider about all of your medical conditions, including if you:
- have prostate or bladder problems, or problems passing urine
- have kidney problems
- are pregnant or plan to become pregnant. It is not known if Qbrexza will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Qbrexza passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with Qbrexza.
Tell your healthcare provider about all the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements.
Qbrexza may affect the way other medicines work causing side effects. Especially tell your healthcare provider if you take anticholinergic medicines.
Know the medicines you take. Keep a list of your medicines with you and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I use Qbrexza?
- Use Qbrexza exactly as your healthcare provider tells you to use it.
- Qbrexza comes as a single-use pre-moistened cloth in individual pouches.
- Qbrexza should be applied to the clean, dry, intact skin, of your underarm areas only. Do not apply Qbrexza to broken skin. Do not cover the treated area with a plastic (occlusive) dressing.
- Apply Qbrexza to both underarm areas using 1 cloth 1 time every 24 hours.
Applying Qbrexza:
- Carefully tear open the pouch to avoid tearing the Qbrexza cloth.
- Unfold the Qbrexza cloth and apply Qbrexza by wiping across 1 entire underarm 1 time. Using the same Qbrexza cloth, wipe across the other underarm 1 time.
- Throw away (discard) the used Qbrexza cloth in the trash.
- Wash your hands right away after you apply Qbrexza and have thrown away the cloth. It is important that you wash your hands because the Qbrexza that is still on your hands can cause you to have blurred vision if you touch your eyes.
- Do not reuse the Qbrexza cloth.
What should I avoid while using Qbrexza?
- Qbrexza may cause you to have blurred vision that is temporary. If you develop blurred vision, call your healthcare provider, stop using Qbrexza and do not drive, operate machinery, or do hazardous work until your vision is clear.
- Qbrexza is flammable. Avoid heat and flame while applying Qbrexza to your skin
What are the possible side effects of Qbrexza?
Qbrexza can cause serious side effects, including:
-
New or worsened urinary retention. People who use Qbrexza may develop new or worse urinary retention. Urinary retention can be caused by a blockage in your bladder. Urinary retention can also happen in men who have a larger than normal prostate. Symptoms of urinary retention may include:
- difficulty urinating
- urinating frequently
- urination in a weak stream or drips
- full bladder or difficulty emptying your bladder (distended bladder)
If you have these symptoms, stop using Qbrexza and call your healthcare provider right away.
-
Problems with control of your body temperature. Qbrexza can cause you to have decreased sweating in areas other than the underarm area which could cause you to become overheated and to develop heat illness. When in hot or very warm temperatures, watch for lack of sweating on your body (generalized) and stop using Qbrexza if you develop lack of sweating on your body.
Stop using Qbrexza and call your healthcare provider right away if you develop any of these symptoms of heat illness:
- hot, red skin
- decreased alertness or passing out (unconsciousness)
- fast, weak pulse
- fast, shallow breathing
- increased body temperature (fever)
-
Blurred vision. If you develop blurred vision during treatment with Qbrexza, call your healthcare provider, stop using Qbrexza and do not drive, or operate machinery, or do hazardous work until your vision is clear.
The most common side effects of Qbrexza include:- dry mouth
- dilation of the pupils of your eyes (mydriasis)
- sore throat
- skin redness, burning/stinging or itching in underarm area
- headache
- problems with urination
- blurred vision
- nasal dryness
- throat, eye, and skin dryness
- constipation
These are not all of the possible side effects of Qbrexza.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088
How should I store Qbrexza?
- Store Qbrexza at room temperature between 68°F and 77°F (20°C and 25°C).
- Qbrexza is flammable. Keep Qbrexza away from heat and flame.
Keep Qbrexza and all medicines out of the reach of children.
General information about the safe and effective use of Qbrexza.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use Qbrexza for a condition for which it was not prescribed. Do not give Qbrexza to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Qbrexza that is written for health professionals.
What are the ingredients in Qbrexza?
Active Ingredient: glycopyrronium tosylate
Inactive Ingredients: citric acid, dehydrated alcohol, purified water, and sodium citrate
Manufactured for: Dermira, Inc. Menlo Park, California 94025
For more information, go to www.Qbrexza.com or call 1-877-337-5553.
-
PRINCIPAL DISPLAY PANEL - NDC: 70428-011-11 - 1-Count Pouch Label
NDC: 70428-011-11
Rx Only
Each pouch contains 1 cloth with 2.8 grams of solution
1 Single-use Cloth
Qbrexza™
(glycopyrronium) cloth
2.4%Manufactured for:
Dermira, Inc., Menlo Park, CA 94025
-
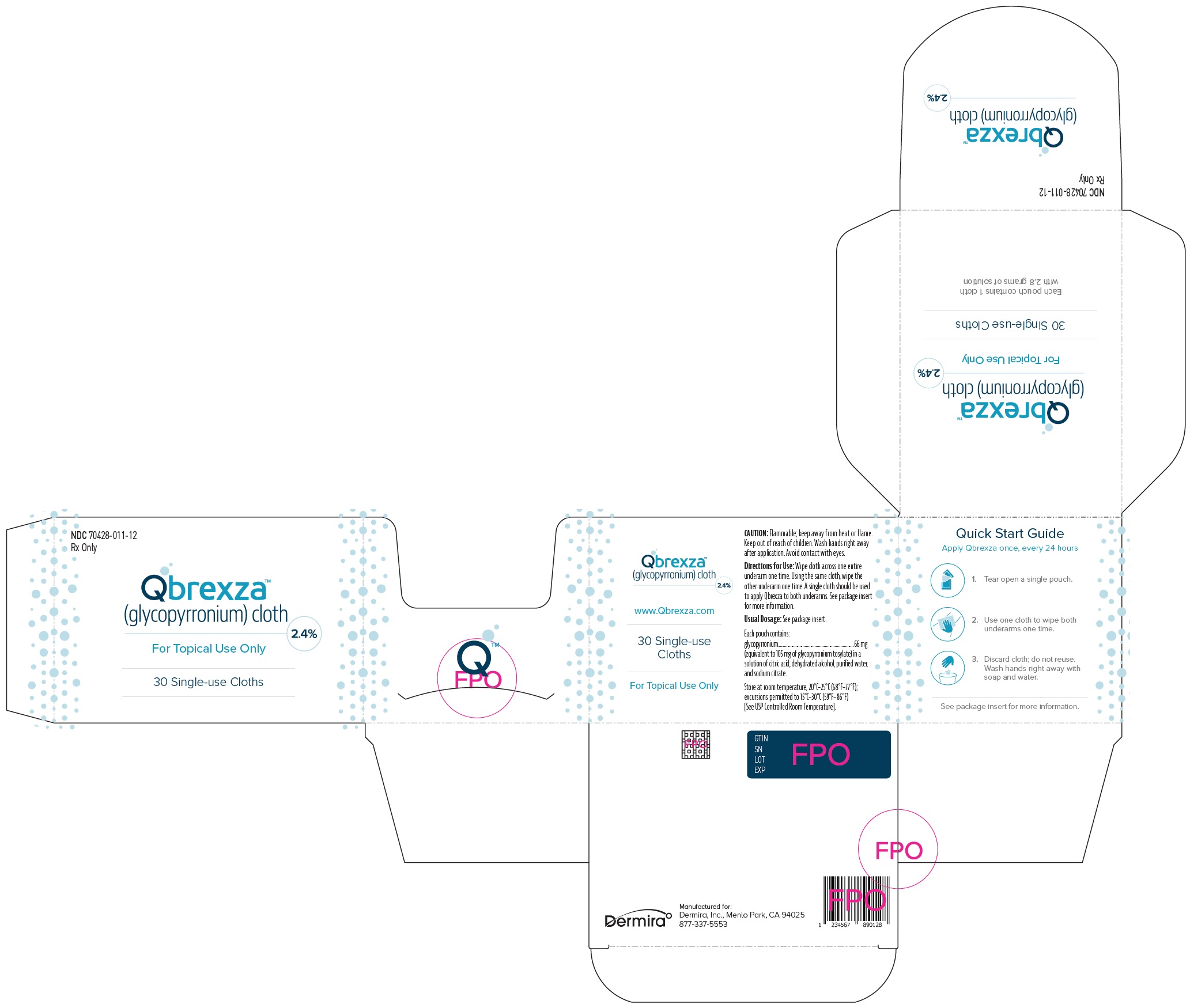
PRINCIPAL DISPLAY PANEL - NDC: 70428-011-12 - 30-Count Carton Label
NDC: 70428-011-12
Rx OnlyQbrexza™
(glycopyrronium)
cloth 2.4%30 Single-use Cloths
Each pouch contains: glycopyrronium 66 mg
For topical use only.
-
INGREDIENTS AND APPEARANCE
QBREXZA
glycopyrronium clothProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 70428-011 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GLYCOPYRRONIUM TOSYLATE (UNII: 1PVF6JLU7B) (GLYCOPYRRONIUM - UNII:A14FB57V1D) GLYCOPYRRONIUM 2.4 g in 100 g Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70428-011-12 30 in 1 CARTON 06/28/2018 1 NDC: 70428-011-11 2.8 g in 1 POUCH; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA210361 06/28/2018 Labeler - Dermira, Inc. (035946260)
Trademark Results [Qbrexza]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 QBREXZA 87745446 5639520 Live/Registered |
Dermira, Inc. 2018-01-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.