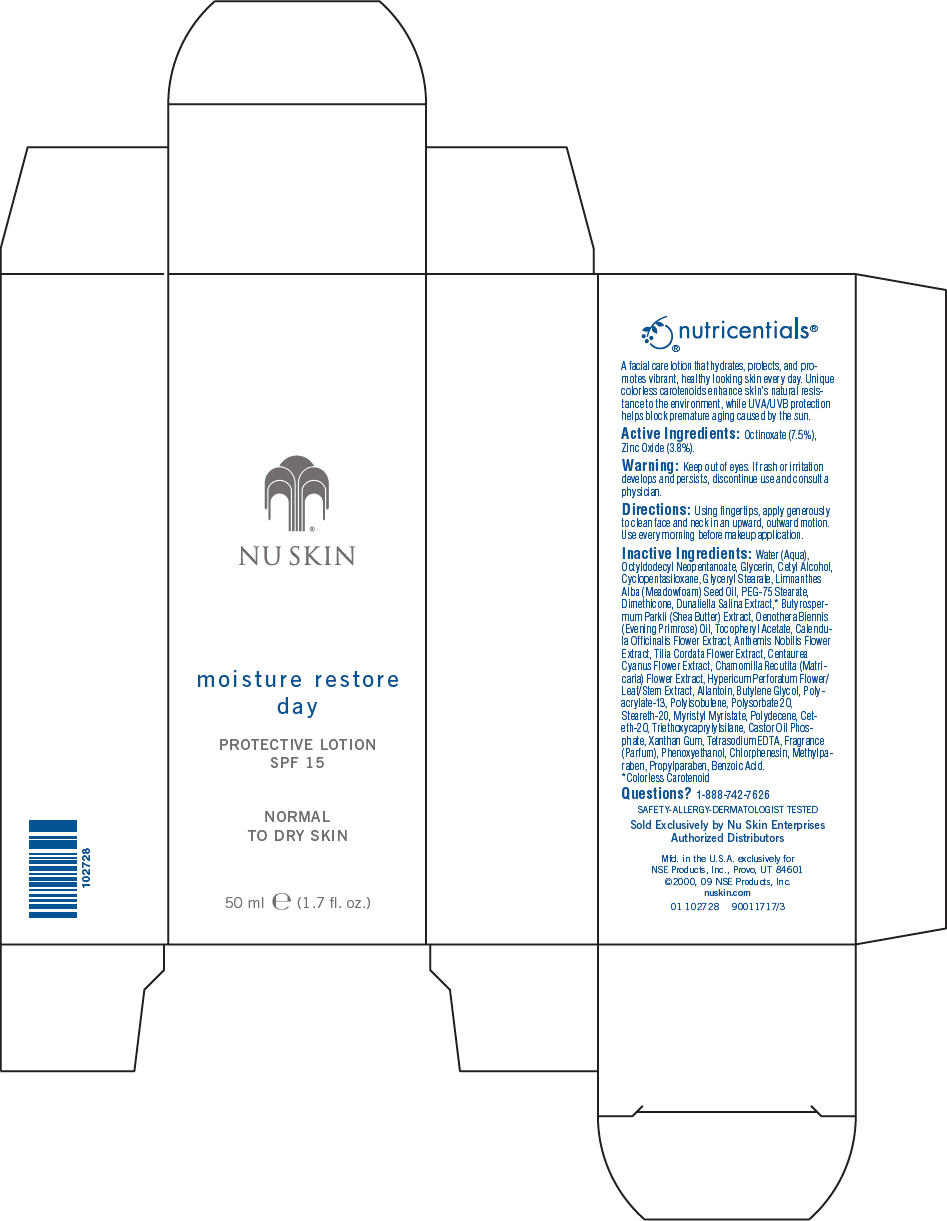
NU SKIN MOISTURE RESTORE DAY PROTECTIVE SPF 15 NORMAL TO DRY SKIN- octinoxate and titanium dioxide lotion
Nu Skin by
Drug Labeling and Warnings
Nu Skin by is a Otc medication manufactured, distributed, or labeled by NSE Products, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredients
- Warning
- Directions
-
Inactive Ingredients
Water (Aqua), Octyldodecyl Neopentanoate, Glycerin, Cetyl Alcohol, Cyclopentasiloxane, Glyceryl Stearate, Limnanthes Alba (Meadowfoam) Seed Oil, PEG-75 Stearate, Dimethicone, Dunaliella Salina Extract,1 Butyrospermum Parkii (Shea Butter) Extract, Oenothera Biennis (Evening Primrose) Oil, Tocopheryl Acetate, Calendula Officinalis Flower Extract, Anthemis Nobilis Flower Extract, Tilia Cordata Flower Extract, Centaurea Cyanus Flower Extract, Chamomilla Recutita (Matricaria) Flower Extract, Hypericum Perforatum Flower/Leaf/Stem Extract, Allantoin, Butylene Glycol, Polyacrylate-13, Polyisobutene, Polysorbate 20, Steareth-20, Myristyl Myristate, Polydecene, Ceteth-20, Triethoxycaprylylsilane, Castor Oil Phosphate, Xanthan Gum, Tetrasodium EDTA, Fragrance (Parfum), Phenoxyethanol, Chlorphenesin, Methylparaben, Propylparaben, Benzoic Acid.
- 1 Colorless Carotenoid
- Questions?
- PRINCIPAL DISPLAY PANEL - 50 ml Carton
-
INGREDIENTS AND APPEARANCE
NU SKIN MOISTURE RESTORE DAY PROTECTIVE SPF 15 NORMAL TO DRY SKIN
octinoxate and titanium dioxide lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 62839-2728 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Octinoxate (UNII: 4Y5P7MUD51) (Octinoxate - UNII:4Y5P7MUD51) Octinoxate 75 g in 1000 mL Titanium Dioxide (UNII: 15FIX9V2JP) (Titanium Dioxide - UNII:15FIX9V2JP) Titanium Dioxide 38 g in 1000 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Octyldodecyl Neopentanoate (UNII: X8725R883T) Glycerin (UNII: PDC6A3C0OX) Cetyl Alcohol (UNII: 936JST6JCN) Cyclomethicone 5 (UNII: 0THT5PCI0R) Glyceryl Monostearate (UNII: 230OU9XXE4) Meadowfoam Seed Oil (UNII: 412ZHA4T4Y) PEG-75 Stearate (UNII: OT38R0N74H) Dimethicone (UNII: 92RU3N3Y1O) Sheanut Oil (UNII: O88E196QRF) Dunaliella Salina (UNII: F4O1DKI9A6) Evening Primrose Oil (UNII: 3Q9L08K71N) Calendula Officinalis Flower (UNII: P0M7O4Y7YD) Chamaemelum Nobile Flower (UNII: O2T154T6OG) Tilia Cordata Flower (UNII: CFN6G1F6YK) Centaurea Cyanus Flower (UNII: QZ239038YC) Chamomile (UNII: FGL3685T2X) St. John's Wort (UNII: UFH8805FKA) Allantoin (UNII: 344S277G0Z) Butylene Glycol (UNII: 3XUS85K0RA) Polysorbate 20 (UNII: 7T1F30V5YH) Steareth-20 (UNII: L0Q8IK9E08) Myristyl Myristate (UNII: 4042ZC00DY) Ceteth-20 (UNII: I835H2IHHX) Triethoxycaprylylsilane (UNII: LDC331P08E) Xanthan Gum (UNII: TTV12P4NEE) Edetate Sodium (UNII: MP1J8420LU) Phenoxyethanol (UNII: HIE492ZZ3T) Chlorphenesin (UNII: I670DAL4SZ) Methylparaben (UNII: A2I8C7HI9T) Propylparaben (UNII: Z8IX2SC1OH) Benzoic Acid (UNII: 8SKN0B0MIM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62839-2728-1 1 in 1 CARTON 06/10/2001 1 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 06/10/2001 Labeler - NSE Products, Inc. (803486393)
Trademark Results [Nu Skin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NU SKIN 97717211 not registered Live/Pending |
NSE Products, Inc. 2022-12-14 |
 NU SKIN 97445076 not registered Live/Pending |
NSE Products, Inc. 2022-06-06 |
 NU SKIN 86503747 4879840 Live/Registered |
NSE Products, Inc. 2015-01-14 |
 NU SKIN 85681553 4268340 Live/Registered |
NSE Products, Inc. 2012-07-19 |
 NU SKIN 85681442 4268338 Live/Registered |
NSE Products, Inc. 2012-07-19 |
 NU SKIN 85114552 3950663 Live/Registered |
NSE Products, Inc. 2010-08-24 |
 NU SKIN 77475815 not registered Dead/Abandoned |
NSE Products, Inc. 2008-05-15 |
 NU SKIN 77442893 not registered Dead/Abandoned |
NSE Products, Inc. 2008-04-08 |
 NU SKIN 73756273 1551560 Live/Registered |
NUSKIN INTERNATIONAL, INC. 1988-10-06 |
 NU SKIN 73671633 not registered Dead/Abandoned |
NU SKIN INTERNATIONAL, INC. 1987-06-25 |
 NU SKIN 73661858 1542160 Live/Registered |
NU SKIN INTERNATIONAL, INC. 1987-05-20 |
 NU SKIN 73542128 1433479 Live/Registered |
NU SKIN INTERNATIONAL, INC. 1985-06-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.