VIRT-PHOS 250 NEUTRAL- sodium phosphate, dibasic, anhydrous, potassium phosphate, monobasic, and sodium phosphate, monobasic, monohydrate tablet
Virt-Phos 250 Neutral by
Drug Labeling and Warnings
Virt-Phos 250 Neutral by is a Other medication manufactured, distributed, or labeled by Virtus Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- STATEMENT OF IDENTITY
-
DESCRIPTION
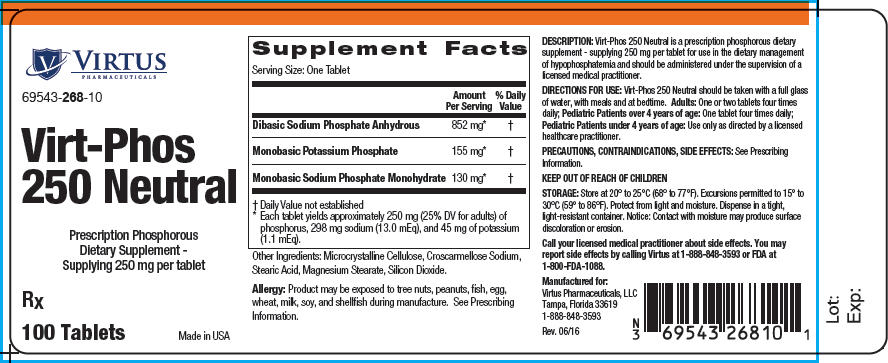
Virt-Phos 250 Neutral is a prescription phosphorous dietary supplement - supplying 250 mg phosphorous per tablet for use in the dietary management of hypophosphatemia and should be administered under the supervision of a licensed medical practitioner.
Supplement Facts Serving Size: One Tablet Amount Per Serving % Daily Value - * Each tablet yields approximately 250 mg (25% DV for adults) of phosphorus, 298 mg sodium (13.0 mEq), and 45 mg of potassium (1.1 mEq).
- † Daily Value not established
Dibasic Sodium Phosphate Anhydrous 852 mg* † Monobasic Potassium Phosphate 155 mg* † Monobasic Sodium Phosphate Monohydrate 130 mg* † Other Ingredients: Microcrystalline Cellulose, Croscarmellose Sodium, Stearic Acid, Magnesium Stearate, Silicon Dioxide.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
PRECAUTIONS
General
This product contains potassium and sodium and should be used with caution if clinical management of these elements is desired. Some individuals may experience a mild laxative effect during the first few days of phosphate therapy; lower the daily dose until this effect subsides or, if necessary, discontinue the use of the product. Caution should be exercised when prescribing this product in the following conditions: Cardiac disease (particularly in patients receiving digitalis); severe adrenal insufficiency (Addison's disease); acute dehydration; severe renal insufficiency; renal function impairment or chronic renal disease; extensive tissue breakdown (such as with severe burns); myotonia congenita; cardiac failure; cirrhosis of the liver or severe hepatic disease; peripheral or pulmonary edema; hypernatremia; hypertension; toxemia of pregnancy; hypoparathyroidism; and acute pancreatitis. High serum phosphate levels may increase the incidence of extraskeletal calcification.
Information for Patients
Patients with kidney stones may pass old stones when phosphate therapy is started and should be warned of this possibility. Patients should be advised to avoid the use of antacids containing aluminum, magnesium, or calcium because they may prevent the absorption of phosphate.
Laboratory Tests
Careful monitoring of renal function and serum calcium, phosphorus, potassium, and sodium may be required at periodic intervals during phosphate therapy. Other tests may be warranted in some patients, depending on conditions.
Drug Interactions
The use of antacids containing magnesium, aluminum, or calcium in conjunction with phosphate preparations may bind the phosphate and prevent its absorption. Concurrent use of antihypertensive drugs or corticosteroids with sodium phosphate may result in hypernatremia. Calcium-containing preparations and/or Vitamin D may antagonize the effects of phosphates in the treatment of hypercalcemia. Potassium-containing medication or potassium-sparing diuretics may cause hyperkalemia. Patients should have serum potassium level determinations at periodic intervals.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No long term or reproduction studies in animals or humans have been performed with Virt-Phos 250 Neutral to evaluate its carcinogenic, mutagenic, or impairment of fertility potential.
Pregnancy and Lactation
Animal reproduction studies have not been conducted with Virt-Phos 250 Neutral. It is also not known whether this product can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. This product should be given to a pregnant woman only if clearly needed.
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when this product is administered to a nursing woman.
SIDE EFFECTS
Gastrointestinal upset (diarrhea, nausea, stomach pain, or vomiting) may occur with phosphate therapy. Bone and joint pain and possibly osteomalacia could occur.
Adverse effects due to sodium or potassium may be observed: headache; dizziness; mental confusion; seizures; weakness or heaviness of legs; unusual tiredness or weakness; numbness, tingling, pain or weakness of hands or feet; numbness or tingling around lips; fast or irregular heartbeat; shortness of breath or troubled breathing; swelling of feet or lower legs; unusual weight gain; low urine output; unusual thirst.
- DIRECTIONS FOR USE
- STORAGE
-
HOW SUPPLIED
Virt-Phos 250 Neutral is supplied as white tablet, imprinted "V268" dispensed in bottles of 100 tablets.
69543-268-10
This product may - under certain circumstances, be dispensed through a certified mail-order program so long as there is record of prescription AND confirmation that the patient is under licensed medical supervision.
-
SAFE HANDLING WARNING
KEEP THIS OUT OF REACH OF CHILDREN
All prescriptions using this product shall be pursuant to state statutes as applicable. This is not an Orange Book product.
Call your licensed medical practitioner about side effects. You may report side effects by calling Virtus at 1-888-848-3593 or FDA at 1-800-FDA-1088.
- HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 100 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
VIRT-PHOS 250 NEUTRAL
sodium phosphate, dibasic, anhydrous, potassium phosphate, monobasic, and sodium phosphate, monobasic, monohydrate tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:69543-268 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Sodium Phosphate, Dibasic, Anhydrous (UNII: 22ADO53M6F) (Phosphate Ion - UNII:NK08V8K8HR, Sodium Cation - UNII:LYR4M0NH37) Sodium Phosphate, Dibasic, Anhydrous 852 mg Potassium Phosphate, Monobasic (UNII: 4J9FJ0HL51) (Phosphate Ion - UNII:NK08V8K8HR, Potassium Cation - UNII:295O53K152) Potassium Phosphate, Monobasic 155 mg Sodium Phosphate, Monobasic, Monohydrate (UNII: 593YOG76RN) (Phosphate Ion - UNII:NK08V8K8HR, Sodium Cation - UNII:LYR4M0NH37) Sodium Phosphate, Monobasic, Monohydrate 130 mg Inactive Ingredients Ingredient Name Strength Cellulose, Microcrystalline (UNII: OP1R32D61U) Croscarmellose Sodium (UNII: M28OL1HH48) Magnesium Stearate (UNII: 70097M6I30) Silicon Dioxide (UNII: ETJ7Z6XBU4) Stearic Acid (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:69543-268-10 100 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 07/17/2015 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 19 mm imprint Labeler - Virtus Pharmaceuticals (079659493)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.