Mydriatic-3 by Imprimis NJOF LLC
Mydriatic-3 by
Drug Labeling and Warnings
Mydriatic-3 by is a Prescription medication manufactured, distributed, or labeled by Imprimis NJOF LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
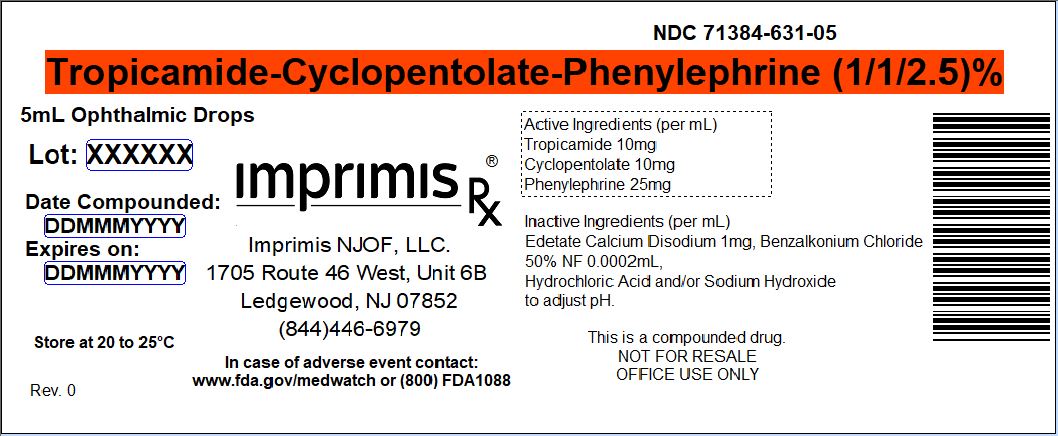
MYDRIATIC-3- tropicamide - cyclopentolate - phenylephrine solution/ drops
Imprimis NJOF LLC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
| MYDRIATIC-3
tropicamide - cyclopentolate - phenylephrine solution/ drops |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Imprimis NJOF LLC (080431967) |
Revised: 2/2020
Document Id: 9e631dc3-1d21-1193-e053-2a95a90af06d
Set id: 6bb82e43-57f6-172e-e053-2991aa0a2e6e
Version: 2
Effective Time: 20200212
Imprimis NJOF LLC