Signature Care 44-773-Delisted
Laxative by
Drug Labeling and Warnings
Laxative by is a Otc medication manufactured, distributed, or labeled by Better Living Brands, LLC, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
LAXATIVE- sennosides tablet, film coated
Better Living Brands, LLC
----------
Signature Care 44-773-Delisted
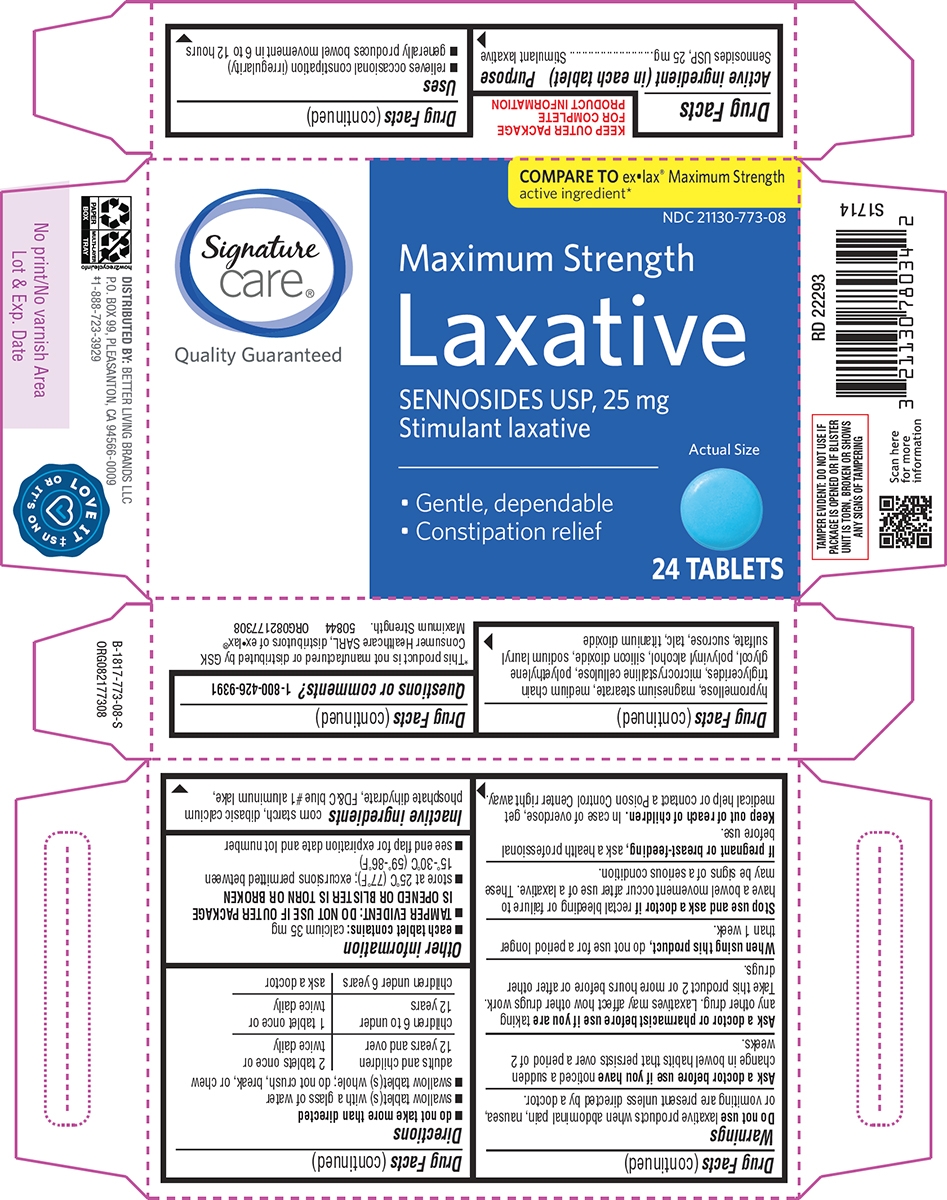
Uses
- relieves occasional constipation (irregularity)
- generally produces bowel movement in 6 to 12 hours
Warnings
Do not use
laxative products when abdominal pain, nausea, or vomiting are present unless directed by a doctor.
Ask a doctor before use if you have
noticed a sudden change in bowel habits that persists over a period of 2 weeks.
Ask a doctor or pharmacist before use if you are
taking any other drug. Laxatives may affect how other drugs work. Take this product 2 or more hours before or after other drugs.
Directions
- do not take more than directed
- swallow tablet(s) with a glass of water
- swallow tablet(s) whole; do not crush, break, or chew
| adults and children 12 years and over | 2 tablets once or twice daily |
| children 6 to under 12 years | 1 tablet once or twice daily |
| children under 6 years | ask a doctor |
Other information
- each tablet contains: calcium 35 mg
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- see end flap for expiration date and lot number
Inactive ingredients
corn starch, dibasic calcium phosphate dihydrate, FD&C blue #1 aluminum lake, hypromellose, magnesium stearate, medium chain triglycerides, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, silicon dioxide, sodium lauryl sulfate, sucrose, talc, titanium dioxide
Principal display panel
Signature
care®
Quality Guaranteed
COMPARE TO exlax® Maximum Strength
active ingredient*
NDC: 21130-773-08
Maximum Strength
Laxative
SENNOSIDES USP, 25 mg
Stimulant laxative
Gentle, dependable
Constipation relief
Actual Size
24 TABLETS
TAMPER EVIDENT: DO NOT USE IF
PACKAGE IS OPENED OR IF BLISTER
UNIT IS TORN, BROKEN OR SHOWS
ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by GSK
Consumer Healthcare SARL, distributors of exlax®
Maximum Strength. 50844 ORG082177308
DISTRIBUTED BY: BETTER LIVING BRANDS LLC
P.O. BOX 99, PLEASANTON, CA 94566-0009
‡1-888-723-3929
LOVE IT
OR IT'S ON US‡
Signature Care 44-773
| LAXATIVE
sennosides tablet, film coated |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Better Living Brands, LLC (009137209) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 038154464 | pack(21130-773) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867837 | manufacture(21130-773) , pack(21130-773) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 832867894 | manufacture(21130-773) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 967626305 | pack(21130-773) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| LNK International, Inc. | 117025878 | manufacture(21130-773) | |