LINCOMIX- lincomycin injection
Lincomix by
Drug Labeling and Warnings
Lincomix by is a Animal medication manufactured, distributed, or labeled by Zoetis Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
INDICATIONS FOR SWINE
LINCOMIX Injectable is indicated for the treatment of infectious forms of arthritis caused by organisms sensitive to its activity. This includes most of the organisms responsible for the various infectious arthritides in swine, such as the staphylococci, streptococci, Erysipelothrix and Mycoplasma spp. It is also indicated for the treatment of mycoplasma pneumonia.
- CONTRAINDICATIONS
- WARNING
- CAUTION
-
ADVERSE REACTIONS
The intramuscular administration to swine may cause a transient diarrhea or loose stools. Although this effect has rarely been reported, one must be alert to the possibility that it may occur. Should this occur, it is important that the necessary steps be taken to prevent the effects of dehydration.
-
DOSAGE AND ADMINISTRATION
For arthritis or mycoplasma pneumonia—5 mg per pound of body weight intramuscularly once daily for three to seven days as needed. When using LINCOMIX Injectable containing 100 mg/mL, 1 mL/20 lb body weight will provide 5 mg/lb. When using LINCOMIX Injectable containing 300 mg/mL, 1 mL/60 lb body weight will provide 5 mg/lb.
For optimal results, initiate treatment as soon as possible.
As with any multi-dose vial, practice aseptic techniques in withdrawing each dose. Adequately clean and disinfect the vial closure prior to entry with a sterile needle and syringe. No vial closure should be entered more than 20 times.
-
HOW SUPPLIED
LINCOMIX Injectable is available in two concentrations: 300 mg/mL and 100 mg/mL.
300 mg/mL: For use in swine weighing 300 pounds or more. Each mL contains lincomycin hydrochloride equivalent to lincomycin, 300 mg; also Benzyl Alcohol, 9.45 mg added as preservative. Supplied in 100 mL vials.
100 mg/mL: Each mL contains lincomycin hydrochloride equivalent to lincomycin, 100 mg; also Benzyl Alcohol, 9.45 mg added as preservative. Supplied in 100 mL vials.
Store at controlled room temperature 20°-25°C (68°- 77°F), with excursions between 15°- 40°C (59°- 104°F).
Use contents within 28 days of first vial broach.
NADA #34-025, Approved by FDA
Zoetis
Distributed by:
Zoetis Inc.
Kalamazoo, MI 49007
Revised: June 2017
Made in Spain -
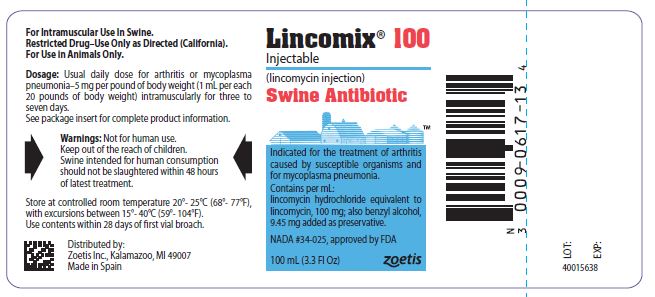
PRINCIPAL DISPLAY PANEL - 100 mg Vial Label
Lincomix 100
Injectable
lincomycin injection, USP
Swine Antibiotic
Indicated for the treatment of arthritis caused
by susceptible organisms and for
mycoplasma pneumonia.
Contains per mL:
lincomycin hydrochloride equivalent to
lincomycin, 100 mg; also benzyl alcohol,
9.45 mg added as preservative.
NADA #34-025, approved by FDA
100 mL (3.3 Fl Oz)
Lincomix Injectable 100 label
-
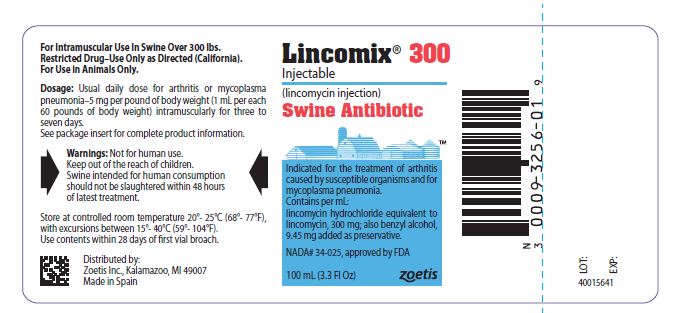
PRINCIPAL DISPLAY PANEL - 300 mg vial label
Lincomix 300
Injectable
lincomycin injection, USP
Swine Antibiotic
Indicated for the treatment of arthritis caused
by susceptible organisms and for
mycoplasma pneumonia.
Contains per mL:
lincomycin hydrochloride equivalent to
lincomycin, 300 mg; also benzyl alcohol,
9.45 mg added as preservative.
NADA #34-025, approved by FDA
100 mL (3.3 Fl Oz)
Lincomix Injectable 300 label
-
INGREDIENTS AND APPEARANCE
LINCOMIX
lincomycin injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 54771-0617 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LINCOMYCIN HYDROCHLORIDE (UNII: M6T05Z2B68) (LINCOMYCIN - UNII:BOD072YW0F) LINCOMYCIN 100 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 9.45 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-0617-1 100 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA034025 06/06/1967 LINCOMIX
lincomycin injectionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 54771-3256 Route of Administration INTRAMUSCULAR Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LINCOMYCIN HYDROCHLORIDE (UNII: M6T05Z2B68) (LINCOMYCIN - UNII:BOD072YW0F) LINCOMYCIN 300 mg in 1 mL Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) 9.45 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54771-3256-1 100 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA034025 06/06/1967 Labeler - Zoetis Inc. (828851555)
Trademark Results [Lincomix]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LINCOMIX 72238321 0827777 Live/Registered |
UPJOHN COMPANY, THE 1966-02-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.