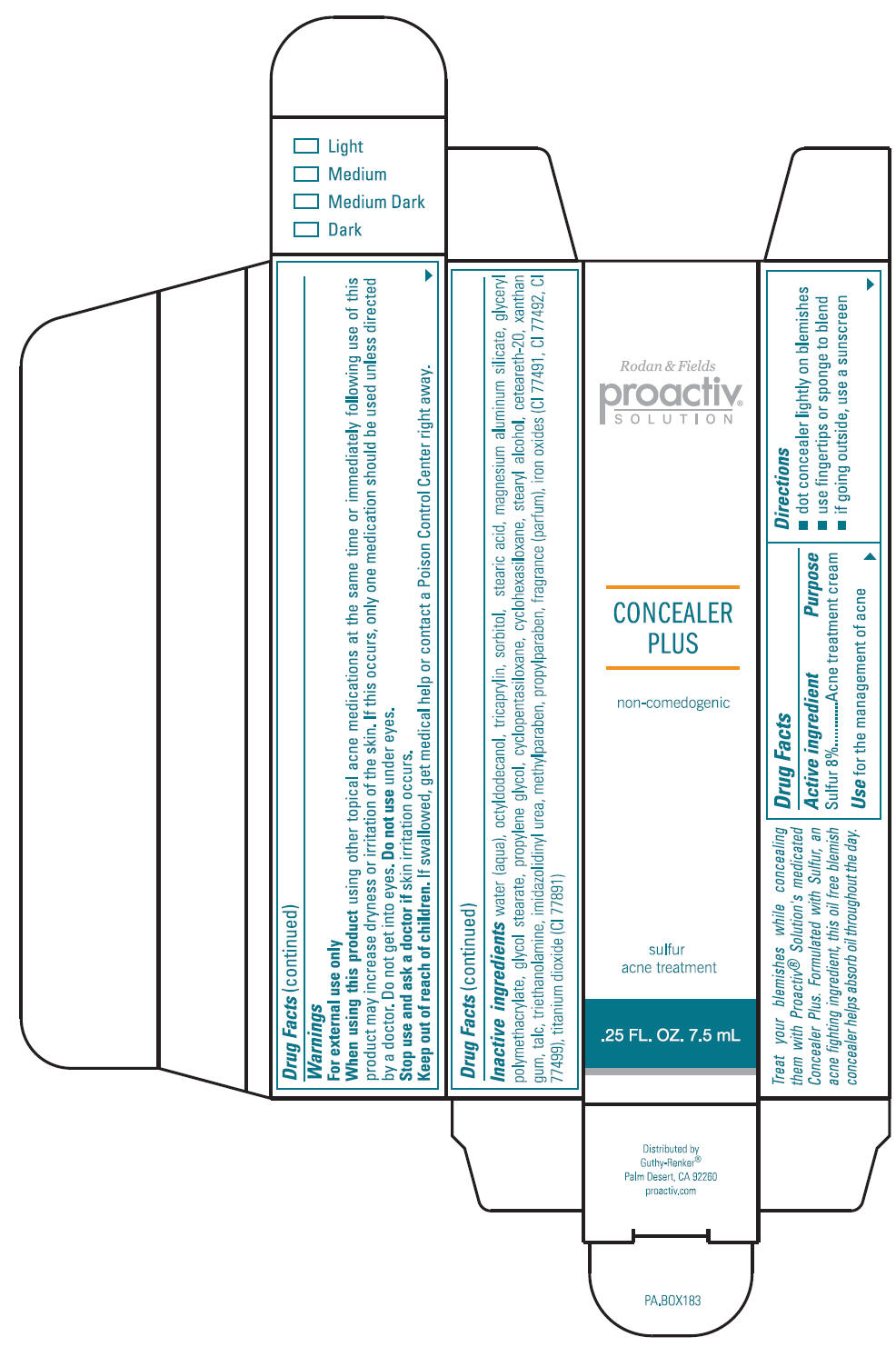
Proactiv Solution Concealer Plus
Proactiv by
Drug Labeling and Warnings
Proactiv by is a Otc medication manufactured, distributed, or labeled by Guthy-Renker LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
PROACTIV CONCEALER PLUS- sulfur lotion
THE PROACTIV COMPANY LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Proactiv Solution Concealer Plus
Directions
- dot concealer lightly on blemishes
- use fingertips or sponge to blend
- if going outside, use a sunscreen
Warnings
For external use only
Inactive Ingredients
water (aqua), octyldodecanol, tricaprylin, sorbitol, stearic acid, magnesium aluminum silicate, glyceryl polymethacrylate, glycol stearate, propylene glycol, cyclopentasiloxane, cyclohexasiloxane, stearyl alcohol, ceteareth-20, xanthan gum, talc, triethanolamine, imidazolidinyl urea, methylparaben, propylparaben, fragrance (parfum), iron oxides (CI 77491, CI 77492, CI 77499), titanium dioxide (CI 77891)
| PROACTIV
CONCEALER PLUS
sulfur lotion |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - THE PROACTIV COMPANY LLC (080216357) |
| Registrant - THE PROACTIV COMPANY LLC (080216357) |
Trademark Results [Proactiv]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 PROACTIV 85171884 not registered Dead/Abandoned |
THE PROACTIV COMPANY SÃRL 2010-11-08 |
 PROACTIV 85023382 4143243 Dead/Cancelled |
THE PROACTIV COMPANY SÃRL 2010-04-26 |
 PROACTIV 78439806 not registered Dead/Abandoned |
AVO Multi-Amp Corporation, doing business as Megger 2004-06-23 |
 PROACTIV 78292301 not registered Dead/Abandoned |
VFS TECHNOLOGIES LIMITED 2003-08-26 |
 PROACTIV 78259207 not registered Dead/Abandoned |
ACTIV Financial Systems, Inc. 2003-06-06 |
 PROACTIV 76441664 not registered Dead/Abandoned |
E. Excel International, Inc. 2002-08-20 |
 PROACTIV 76149463 not registered Dead/Abandoned |
InfoActiv, Incorporated 2000-10-19 |
 PROACTIV 75930916 not registered Dead/Abandoned |
NATURELAND BIO PRODUCTS LTD 2000-02-29 |
 PROACTIV 75682121 2574142 Live/Registered |
THE PROACTIV COMPANY SÃRL 1999-04-13 |
 PROACTIV 75493592 not registered Dead/Abandoned |
Knightlite (U.K.) Ltd. 1998-05-29 |
 PROACTIV 74586544 2162306 Live/Registered |
THE PROACTIV COMPANY SÃRL 1994-10-17 |
 PROACTIV 74244642 not registered Dead/Abandoned |
For Women Only, Inc. 1992-02-10 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.