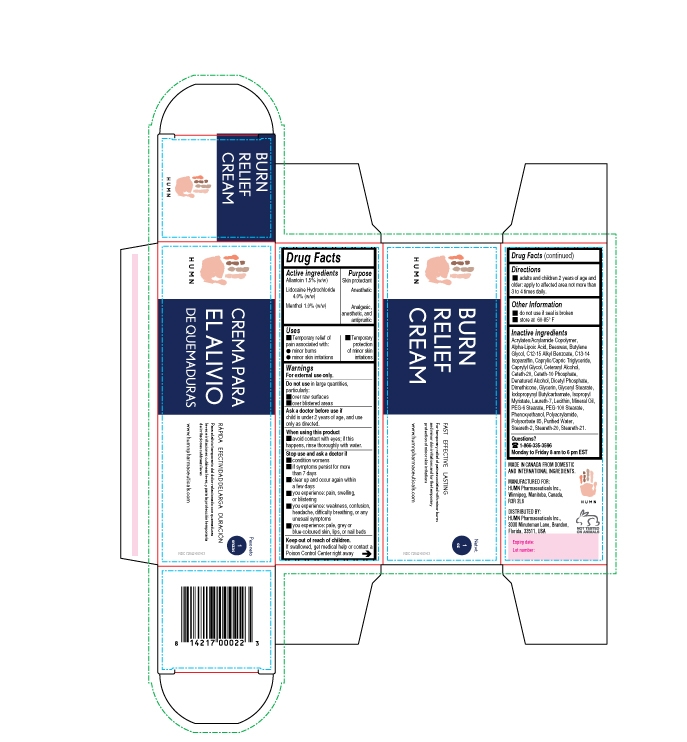
BURN RELIEF- allantoin, lidocaine, menthol cream
Burn Relief by
Drug Labeling and Warnings
Burn Relief by is a Otc medication manufactured, distributed, or labeled by HUMN Pharmaceuticals Inc, Delta Pharma Inc, Delta Pharma Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- DO NOT USE
- ASK DOCTOR
- WHEN USING
-
STOP USE
Stop use and ask a doctor
condition worsens if symptoms persist for more than 7 days
clear up and occur again within a few days you experience: pain, swelling, or blistering
you experience: weakness, confusion, headache, difficulty breathing, or any unusual symptoms
you experience: pale, grey or blue-coloured skin, lips, or nail beds
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INFORMATION FOR PATIENTS
-
INACTIVE INGREDIENT
Inactive ingredients
Acrylates/Acrylamide Copolymer, Alpha-Lipoic Acid, Beeswax, Butylene Glycol, C12-15 Alkyl Benzoate, C13-14 Isoparaffin, Caprylic/Capric Triglyceride, Caprylyl Glycol, Ceteraryl Alcohol, Ceteth-20, Ceteth-10 Phosphate, Denatured Alcohol, Dicetyl Phosphate, Dimethicone, Glycerin, Glyceryl Stearate, Iodopropynyl Butylcarbamate, Isopropyl Myristate, Laureth-7, Lecithin, Mineral Oil, PEG-6 Stearate, PEG-100 Stearate, Phenoxyethanol, Polyacrylamide, Polysorbate 85, Purified Water, Steareth-2, Steareth-20, Steareth-21.
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BURN RELIEF
allantoin, lidocaine, menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72042-003 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 1 mg in 100 mg LIDOCAINE HYDROCHLORIDE ANHYDROUS (UNII: EC2CNF7XFP) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 4 mg in 100 mg ALLANTOIN (UNII: 344S277G0Z) (ALLANTOIN - UNII:344S277G0Z) ALLANTOIN 1.5 mg in 100 mg Inactive Ingredients Ingredient Name Strength CETETH-20 (UNII: I835H2IHHX) ALCOHOL (UNII: 3K9958V90M) ALUMINUM DICETYL PHOSPHATE (UNII: WMV3R5DS7O) GLYCERIN (UNII: PDC6A3C0OX) MINERAL OIL (UNII: T5L8T28FGP) PEG-6 STEARATE (UNII: 8LQC57C6B0) PEG-100 STEARATE (UNII: YD01N1999R) POLYACRYLAMIDE (10000 MW) (UNII: E2KR9C9V2I) STEARETH-2 (UNII: V56DFE46J5) STEARETH-20 (UNII: L0Q8IK9E08) CETETH-10 PHOSPHATE (UNII: 4E05O5N49G) PEG-120 GLYCERYL STEARATE (UNII: 6941286E4I) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) LAURETH-7 (UNII: Z95S6G8201) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIMETHICONE 100 (UNII: RO266O364U) POLYSORBATE 85 (UNII: A7F3N56197) WATER (UNII: 059QF0KO0R) CAPRYLYL GLYCOL (UNII: 00YIU5438U) CAPRYLIC/CAPRIC/LAURIC TRIGLYCERIDE (UNII: FJ1H6M2JG9) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) ACRYLAMIDE (UNII: 20R035KLCI) CETYL ALCOHOL (UNII: 936JST6JCN) YELLOW WAX (UNII: 2ZA36H0S2V) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) .ALPHA.-LIPOIC ACID (UNII: 73Y7P0K73Y) STEARETH-21 (UNII: 53J3F32P58) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72042-003-03 1 in 1 CARTON 05/19/2018 1 28300 mg in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/16/2018 Labeler - HUMN Pharmaceuticals Inc (245630272) Registrant - Delta Pharma Inc (200161730) Establishment Name Address ID/FEI Business Operations Delta Pharma Inc. 200161730 manufacture(72042-003)
Trademark Results [Burn Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BURN RELIEF 74233285 1848480 Live/Registered |
O-TWO MEDICAL TECHNOLOGIES INC. 1991-12-24 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.