OPTIRAY 350- ioversol injection OPTIRAY 320- ioversol injection OPTIRAY 300- ioversol injection
Optiray by
Drug Labeling and Warnings
Optiray by is a Prescription medication manufactured, distributed, or labeled by Liebel-Flarsheim Company LLC, LIEBEL-FLARSHEIM COMPANY LLC, SpecGx LLC, Guerbet Ireland Unlimited Company. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use OPTIRAY PHARMACY BULK PACKAGE safely and effectively. See full prescribing information for OPTIRAY.
OPTIRAY® (ioversol) Injection for intravenous use
Initial U.S. Approval: 1988WARNING: NOT FOR INTRATHECAL USE
See full prescribing information for complete boxed warning.
Inadvertent intrathecal administration may cause death, convulsion, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. (5.1)RECENT MAJOR CHANGES
Warnings and Precautions (5.10) 3/2017
INDICATIONS AND USAGE
OPTIRAY is a radiographic contrast agent indicated for the following:
Intra-arterial Procedures (1.1)
Adults:- Cerebral Arteriography (300, 320 mg iodine/mL)
- Peripheral Arteriography (300, 320, 350 mg iodine/mL )
- Visceral and Renal Arteriography, Aortography (320 mg iodine/mL)
- Coronary Arteriography and Left Ventriculography (320, 350 mg iodine/mL)
Pediatric Patients: Angiocardiography (320, 350 mg iodine/mL)
Intravenous Procedures (1.2)
Adults:- Computed tomography (CT) Imaging of Head and Body (300, 320, 350 mg iodine/mL)
- Venography (300, 320, 350 mg iodine/mL)
- Intravenous Excretory Urography (300, 320, 350 mg iodine/mL)
- Intravenous Digital Subtraction Angiography (350 mg iodine/mL)
Pediatric Patients: CT Imaging of the Head and Body, and Intravenous Excretory Urography (320mg iodine/mL).
DOSAGE AND ADMINISTRATION
The Pharmacy Bulk Package is not for direct infusion.
Adjust the volume and concentration of Optiray. Modify the dose accounting for factors such as age, body weight, vessel size, blood flow rate within the vessel. Please see details in full Prescribing Information. (2)
DOSAGE FORMS AND STRENGTHS
Optiray Pharmacy Bulk Package is available in three strengths:
Injection: 300 mg iodine/mL (ioversol 64%), 320 mg iodine/mL (ioversol 68%), 350 mg iodine/mL (ioversol 74%) in pharmacy bulk package bottles. (3)CONTRAINDICATIONS
Symptomatic Hyperthyroidism (4)
WARNINGS AND PRECAUTIONS
- Hypersensitivity Reactions: life-threatening or fatal reactions can occur. Always have emergency equipment and trained personnel available. (5.2)
- Contrast Induced Acute Kidney Injury: Acute injury, including renal failure, can occur. Minimize dose and maintain adequate hydration to minimize risk. (5.3)
- Cardiovascular Reactions: hemodynamic disturbances including shock and cardiac arrest may occur during or after administration. (5.4)
ADVERSE REACTIONS
The most common reaction is nausea, occurring at a rate of greater than 1 percent. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact LIEBEL-FLARSHEIM COMPANY LLC at 855-266-5037 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.USE IN SPECIFIC POPULATIONS
- Lactation: A lactating woman may pump and discard breast milk for 8 hours after Optiray administration. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2017
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Intra-arterial
1.2 Intra-venous
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
2.2 Intra-arterial Procedures in Adults
2.3 Intravenous Procedures in Adults
2.4 Pediatric Dosing
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risks Associated with Inadvertent Intrathecal Administration
5.2 Hypersensitivity Reactions
5.3 Contrast Induced Acute Kidney Injury
5.4 Cardiovascular Adverse Reactions
5.5 Thromboembolic Events
5.6 Extravasation and Injection Site Reactions
5.7 Thyroid Storm in Patients with Hyperthyroidism
5.8 Hypertensive Crisis in Patients with Pheochromocytoma
5.9 Sickle Cell Crisis in Patients with Sickl Cell Disease
5.10 Severe Cutaneous Adverse Reactions
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions
7.2 Drug/Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
10 OVERDOSAGE
11 DESCRIPTION
11.1 Chemical Characteristics
11.2 Physical Characteristics
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: NOT FOR INTRATHECAL USE
Inadvertent intrathecal administration may cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema. (5.1)
-
1 INDICATIONS AND USAGE
Optiray is indicated for:
1.1 Intra-arterial
In adults
- Optiray 300: cerebral arteriography, and peripheral arteriography.
- Optiray 320: cerebral arteriography, peripheral arteriography, visceral and renal arteriography, aortography, coronary arteriography, and left ventriculography.
- Optiray 350: peripheral arteriography, coronary arteriography, and left ventriculography.
In pediatric patients
- Optiray 320 and Optiray 350: angiocardiography.
1.2 Intra-venous
In adults
- Optiray 300: CT imaging of the head and body, venography, and intravenous excretory urography.
- Optiray 320: CT imaging of the head and body, venography, and intravenous excretory urography.
- Optiray 350: CT imaging of the head and body, venography, intravenous excretory urography, and intravenous digital subtraction angiography (IV-DSA).
In pediatric patients
- Optiray 320: CT imaging of the head and body, and intravenous excretory urography.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
- Optiray is for intravascular use only [see Boxed Warning, Contraindications (4), Warnings and Precautions (5.1)].
- Use sterile technique for all handling and administration of Optiray.
- Inspect glass container prior to use for breakage or other damage and do not use damaged containers.
- Warm Optiray and administer at body or room temperature.
- Inspect Optiray for particulate matter or discoloration before administration. Do not administer if Optiray contains particulate matter or is discolored.
- Do not mix Optiray with other drugs, solutions or total parenteral nutrition mixtures.
- Use the lowest dose necessary to obtain adequate visualization.
- Adjust the volume and concentration of Optiray. Modify the dose accounting for factors such as age, body weight, vessel size, blood flow rate within the vessel, anticipated pathology, degree and extent of opacification required, structure(s) or area to be examined, disease processes affecting the patient, and equipment and technique to be employed.
- Avoid extravasation when injecting Optiray; especially in patients with severe arterial or venous disease [see Warnings and Precautions (5.6)].
- Hydrate patients before and after Optiray administration [see Warnings and Precautions (5.3)].
Directions for Proper Use of Optiray Pharmacy Bulk Package
- The Pharmacy Bulk Package is not for direct infusion.
- Penetrate the container closure only one time, utilizing a suitable sterile transfer device or dispensing set which allows measured distribution of the contents.
- Transfer Optiray from the Pharmacy Bulk Package only in a suitable work area, such as a laminar flow hood, utilizing aseptic technique.
- Withdraw container contents immediately. However, should this not be possible, a maximum time of 4 hours from initial closure entry is permitted to complete fluid transfer operations.
- Temperature of container after the closure has been entered should not exceed 25°C (77°F).
2.2 Intra-arterial Procedures in Adults
- Cerebral Arteriography
Use Optiray 300 or Optiray 320. The recommended dose for visualization of cerebral arteries is shown below (may repeat as necessary):
Diagnostic area Dose Maximum Cumulative Dose carotid or vertebral arteries 2 to 12 mL 200 mL aortic arch injection
(four vessel study)
20 to 50 mL 200 mL - Peripheral Arteriography
Use Optiray 300, Optiray 320 or Optiray 350. The recommended dose for visualization of peripheral arteries is shown below (may repeat as necessary):
Diagnostic area Dose Maximum Cumulative Dose aorta-iliac runoff 60 mL (range 20 to 90 mL) 250 mL common iliac, femoral 40 mL (range 10 to 50 mL) 250 mL subclavian, brachial 20 mL (range 15 to 30 mL) 250 mL - Visceral and Renal Arteriography and Aortography
Use Optiray 320. The recommended dose for visualization for the aorta and visceral arteries is shown below (may repeat as necessary):
Diagnostic area Dose Maximum Cumulative Dose aorta 45 mL (range 10 to 80 mL) 250 mL celiac 45 mL (range 12 to 60 mL) 250 mL superior mesenteric 45 mL (range 15 to 60 mL) 250 mL renal or inferior mesenteric 9 mL (range 6 to 15 mL) 250 mL - Coronary Arteriography and Left Ventriculography
Use Optiray 320 or Optiray 350. The recommended dose for visualization of the coronary arteries and left ventricle is shown below (may repeat as necessary):
Diagnostic area Dose Maximum Cumulative Dose left coronary 8 mL (range 2 to 10 mL) 250 mL right coronary 6 mL (range 1 to 10 mL) 250 mL left ventricle 40 mL (range 30 to 50 mL) 250 mL 2.3 Intravenous Procedures in Adults
- Computed Tomography
Use Optiray 300, Optiray 320 or Optiray 350 for head and body imaging.
Head Imaging
The recommended dosing is shown below:- Scan immediately after completion of the intravenous administration.
Infusion
Optiray 300 50 to 150 mL Optiray 320 50 to 150 mL Optiray 350 50 to 150 mL Body Imaging
Optiray may be administered by bolus injection, by rapid infusion, or by a combination of both. The recommended dosing is shown below:- Scanning interval will vary with indication and target organ
Bolus Injection
Infusion
Optiray 300 25 to 75mL 50 to 150 mL Optiray 320 25 to 75mL 50 to 150 mL Optiray 350 25 to 75mL 50 to 150 mL - Venography
Use Optiray 300, Optiray 320 or Optiray 350. The recommended dose is 50 to 100 mL per extremity; with a maximum cumulative dose of 250 mL.
- Intravenous Urography
Use Optiray 350, Optiray 320, or Optiray 300. The recommended dose is shown below:
Usual Dose
High Dose Urography
Maximum Dose
Optiray 300 50 to 75mL 1.6 mL/kg 150 mL Optiray 320 50 to 75mL 1.5 to 2 mL/kg 150 mL Optiray 350 50 to 75mL 1.4 mL/kg 150 mL - Intravenous Digital Subtraction Angiography (IV-DSA)
Use Optiray 350. The recommended dose range per injection is 30 to 50 mL; may repeat as necessary with a maximum cumulative dose of 250mL.
Injection rates will vary depending on the site of catheter placement and vessel size.
- Central catheter injections are usually made at a rate of between 10 and 30 mL/second.
- Peripheral injections are usually made at a rate of between 12 and 20 mL/second.
2.4 Pediatric Dosing
Intra-arterial Procedures
- Angiocardiography
Use Optiray 350 or Optiray 320. The recommended single ventricular dose is 1.25 mL/kg (range 1 mL/kg to 1.5 mL/kg). The maximum cumulative dose is 5 mL/kg up to a maximum total volume of 250 mL.
Intravenous Procedures
- Computed Tomography
Use Optiray 320.
Head and Body Imaging
The recommended dose in pediatric patients is 1.5 mL/kg to 2 mL/kg (range 1 mL/kg to 3 mL/kg).- Intravenous Urography
Use Optiray 320. The recommended dose for pediatric patients is 1 mL/kg to 1.5 mL/kg (range 0.5 mL/kg to 3 mL/kg); with a maximum cumulative dose not exceeding 3 mL/kg.
-
3 DOSAGE FORMS AND STRENGTHS
Injection: clear, colorless to pale yellow solutions containing no undissolved solids, available in the following strengths and multiple-dose containers:
Imaging Product mg of
ioversol
per mLmg of organically
bound iodine
per mLPharmacy Bulk Pack Presentation OPTIRAY 300
(Ioversol 64%)636 300 Yes OPTIRAY 320
(Ioversol 68%)678 320 Yes OPTIRAY 350
(Ioversol 74%)741 350 Yes - 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Risks Associated with Inadvertent Intrathecal Administration
Optiray is indicated for intravascular use only [see Dosage and Administration (2.1)]. Inadvertent intrathecal administration can cause death, convulsions, cerebral hemorrhage, coma, paralysis, arachnoiditis, acute renal failure, cardiac arrest, seizures, rhabdomyolysis, hyperthermia, and brain edema.
5.2 Hypersensitivity Reactions
Optiray can cause life-threatening or fatal hypersensitivity reactions including anaphylaxis and anaphylactic shock. Manifestations include respiratory arrest, laryngospasm, bronchospasm, angioedema, and shock. Most severe reactions develop shortly after the start of the injection (e.g. within 1 to 3 minutes), but delayed reactions may occur. There is an increased risk in patients with a history of a previous reaction to contrast agent, and known allergies (i.e., bronchial asthma, drug, or food allergies), and other hypersensitivities. Premedication with antihistamines or corticosteroids to avoid or minimize possible allergic reactions does not prevent serious life-threatening reactions, but may reduce both their incidence and severity.
Obtain a history of allergy, hypersensitivity, or prior hypersensitivity reactions to iodinated contrast agents. Always have emergency resuscitation equipment and trained personnel available and monitor all patients for hypersensitivity reactions.
5.3 Contrast Induced Acute Kidney Injury
Acute kidney injury, including renal failure, may occur after Optiray administration. Risk factors include: pre-existing renal impairment, dehydration, diabetes mellitus, congestive heart failure, advanced vascular disease, elderly age, concomitant use of nephrotoxic or diuretic medications, multiple myeloma / paraproteinaceous diseases, repetitive and/or large doses of an iodinated contrast agent.
Use the lowest necessary dose of Optiray in patients with renal impairment. Adequately hydrate patients prior to and following Optiray administration. Do not use laxatives, diuretics, or preparatory dehydration prior to Optiray administration.
5.4 Cardiovascular Adverse Reactions
Optiray increases the circulatory osmotic load and may induce acute or delayed hemodynamic disturbances in patients with congestive heart failure, severely impaired renal function, combined renal and hepatic disease, combined renal and cardiac disease, particularly when repetitive or large doses are administered.
Life-threatening or fatal cardiovascular reactions have occurred with the use of Optiray, including cardiac arrest, hypotensive collapse, and shock. Most deaths occur within 10 minutes of injection; with cardiovascular disease as the main underlying factor. Cardiac decompensation, serious arrhythmias, and myocardial ischemia or infarction can occur during coronary arteriography and ventriculography.
Based upon literature reports, deaths from the administration of iodinated contrast agents range from 6.6 per 1 million (0.00066 percent) to 1 in 10,000 patients (0.01 percent). Use the lowest necessary dose of Optiray in patients with congestive heart failure and always have emergency resuscitation equipment and trained personnel available. Monitor all patients for severe cardiovascular reactions.
5.5 Thromboembolic Events
Angiocardiography
Serious, fatal, thromboembolic events causing myocardial infarction and stroke can occur during angiographic procedures with Optiray. During these procedures, increased thrombosis and activation of the complement system occurs. Risk factors for thromboembolic events include: length of procedure, catheter and syringe material, underlying disease state, and concomitant medications.To minimize thromboembolic events use meticulous angiographic technique. Avoid blood remaining in contact with syringes containing Optiray, which increases the risk of clotting. Avoid angiocardiography in patients with homocystinuria because of the risk of inducing thrombosis and embolism [see Clinical Pharmacology (12.2)].
5.6 Extravasation and Injection Site Reactions
Extravasation can occur with Optiray administration, particularly in patients with severe arterial or venous disease and can be associated with pain, hemorrhage and necrosis. Ensure intravascular placement of catheters prior to injection. Monitor patients for extravasation and advise patients to seek medical care for progression of symptoms.
5.7 Thyroid Storm in Patients with Hyperthyroidism
Optiray is contraindicated in patients with symptomatic hyperthyroidism [see Contraindications (4)]. Thyroid storm has occurred following the intravascular use of iodinated radiopaque agents in patients with hyperthyroidism or with an autonomously functioning thyroid nodule. Evaluate the risk in such patients before use of Optiray.
5.8 Hypertensive Crisis in Patients with Pheochromocytoma
Hypertensive crisis has occurred after the use of iodinated radiopaque contrast agents in patient with pheochromocytoma. Closely monitor patients when administering Optiray if pheochromocytoma or catecholamine-secreting paraganglioma is suspected. Inject the minimum amount of Optiray necessary and have measures for treatment of hypertensive crisis readily available.
5.9 Sickle Cell Crisis in Patients with Sickl Cell Disease
Iodinated contrast agents may promote sickling in individuals who are homozygous for sickle cell disease. Hydrate patients prior to and following Optiray administration, use Optiray only if the necessary imaging information cannot be obtained with alternative imaging modalities, and inject the minimum amount necessary.
5.10 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions (SCAR) may develop from 1 hour to several weeks after intravascular contrast agent administration. These reactions include Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN), acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS). Reaction severity may increase and time to onset may decrease with repeat administration of a contrast agent; prophylactic medications may not prevent or mitigate severe cutaneous adverse reactions. Avoid administering Optiray to patients with a history of a severe cutaneous adverse reaction to Optiray.
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Risks Associated with Inadvertent Intrathecal Administration [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.2)]
- Contrast Induced Acute Kidney Injury [see Warnings and Precautions (5.3)]
- Cardiovascular Adverse Reactions [see Warnings and Precautions (5.4)]
- Thromboembolic Events [see Warnings and Precautions (5.5)]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.10)
6.1 Clinical Studies Experience
Adult Patients
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.The following table shows reactions based upon clinical trials with Optiray (ioversol) in 4,187 patients. Adverse reactions are listed by organ system according to clinical importance. More severe reactions are listed before others in a system regardless of incidence. The most common reaction is nausea, occurring at a rate of 1 percent.
Cardiac disorders
Cardiac arrest, myocardial infarction, arrhythmia, atrioventricular block complete, atrioventricular block, nodal rhythm, bradycardia, angina pectoris, palpitationsEar and labyrinth disorders
Vertigo, tinnitusEye disorders
Vision blurred, periorbital edema, conjunctivitisGastrointestinal disorders
Vomiting, abdominal pain, dysphagia, dry mouthGeneral disorders and administration site conditions
Chest pain, pain, injection site pain, injection site hematoma, extravasation, pyrexia, swelling, asthenia, malaise, fatigue, chillsInfections and infestations
RhinitisInjury, poisoning, and procedural complications
Heart injury, vascular pseudoaneurysmInvestigations
Electrocardiogram ST segment depression, blood pressure decreasedMetabolism and nutrition disorders
AcidosisMusculoskeletal and connective tissue disorders
Muscular weakness, muscle spasms, back painNervous system disorders
Cerebral infarction, aphasia, tremor, dizziness, presyncope, headache, paraesthesia, dysgeusiaPsychiatric disorders
Hallucination, visual hallucination, disorientation, anxietyRenal and urinary disorders
Urinary retention, renal pain, polyuriaRespiratory, thoracic, and mediastinal disorders
Laryngeal edema, hypoxia, pulmonary edema, dyspnea, hyperventilation, cough, sneezing,
nasal congestionSkin and subcutaneous tissue disorders
Urticaria, rash, pruritus, swelling face, hyperhidrosis, erythemaVascular disorders
Hypertension, hypotension, arterial spasm, vasospasm, vasodilation, flushingPediatric Patients
In clinical trials involving 311 patients for pediatric angiocardiography, contrast enhanced computed tomographic imaging of the head and body, and intravenous excretory urography; 6% of patients reported an adverse reactions, with the most common adverse reactions being nausea and fever. Adverse reactions reported were similar in quality and frequency to the adverse events reported by adults.6.2 Postmarketing Experience
The following adverse drug reactions have been reported during post-approval use of Optiray. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate frequency.
Cardiac disorders: coronary artery spasm, cyanosis, arrhythmia (ventricular fibrillation, tachycardia, extrasystole), ECG abnormal.
Endocrine disorders: thyroid function tests indicative of hypothyroidism or transient thyroid suppression have been uncommonly reported following iodinated contrast media administration to adult and pediatric patients, including infants, some patients were treated for hypothyroidism.
Eye disorders: temporary blindness, conjunctivitis (including eye irritation, ocular hyperemia, watery eyes).
Gastrointestinal disorders: tongue edema, salivary hypersecretion.
General disorders and administration site conditions: injection site reactions including pain, hemorrhage, and necrosis especially after extravasation [see Warnings and Precautions (5.6)], face edema, feeling hot.
Immune system disorders: hypersensitivity reactions including fatal anaphylactic shock.
Nervous system disorders: seizure, loss of consciousness, somnolence, hypoesthesia, dyskinesia, amnesia.
Respiratory disorders: respiratory arrest, asthma, bronchospasm, laryngeal spasm and obstruction, throat irritation, dysphonia.
Skin and subcutaneous tissue disorders: Reactions range from mild (e.g. rash, erythema, pruritus, urticaria, and skin discoloration) to severe: [e.g. Stevens-Johnson syndrome and toxic epidermal necrolysis (SJS/TEN)], acute generalized exanthematous pustulosis (AGEP) and drug reaction with eosinophilia and systemic symptoms (DRESS).
Vascular Disorders: phlebitis, thrombosis.
-
7 DRUG INTERACTIONS
7.1 Drug-Drug Interactions
- Metformin
In patients with renal impairment, metformin can cause lactic acidosis. Iodinated contrast agents appear to increase the risk of metformin induced lactic acidosis, possibly as a result of worsening renal function. Stop metformin at the time of, or prior to, Optiray administration in patients with an eGFR between 30 and 60 mL/min/1.73 m2; in patients with a history of hepatic impairment, alcoholism or heart failure; or in patients who will be administered intra-arterial iodinated contrast agents. Re-evaluate eGFR 48 hours after the imaging procedure, and reinstitute only after renal function is stable.
- Radioactive Iodine
Administration of iodinated contrast agents may interfere with thyroid uptake of radioactive iodine (I 131) and decrease therapeutic efficacy in patients with carcinoma of the thyroid. The decrease in efficacy lasts for 6-8 weeks.
- Oral Cholecystographic Contrast Agents
Renal toxicity has been reported in patients with liver impairment who were given oral cholecystographic agents followed by intravascular contrast agents. Administration of Optiray should be postponed in patients who have recently received a cholecystographic contrast agent.
7.2 Drug/Laboratory Test Interactions
- Protein-Bound Iodine, Radioactive Iodine Determinations
The results of protein bound iodine and radioactive iodine uptake studies, which depend on iodine estimation, will not accurately reflect thyroid function for up to 16 days following administration of iodinated contrast agent. However, thyroid function tests that do not depend on iodine estimations, e.g., T3 resin uptake and total or free thyroxine (T4) assays are not affected.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Postmarketing data with Optiray use in pregnant women are insufficient to determine if there is a risk of drug-associated adverse developmental outcomes. Ioversol crosses the placenta and reaches fetal tissues in small amounts [see Data]. In animal reproduction studies, no adverse developmental effects were observed following intravenous administration of ioversol to pregnant rats and rabbits at doses 0.35 and 0.71 times, respectively, the maximum recommended human dose.The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of major birth defects, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriages in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Human Data
Literature reports show that ioversol crosses the placenta and is visualized in the digestive tract of exposed infants after birth.Animal Data
Developmental toxicity studies were conducted with ioversol given intravenously at doses of 0, 0.2, 0.8, and 3.2 g iodine/kg/day from Gestation Day7 to 17 and 6 to 18, in rats and rabbits, respectively. No adverse effects on embryo-fetal development were observed in either species at the maximum dose tested (3.2 g iodine/kg/day). Maternal toxicity was observed in rabbits at 0.8 and 3.2 g iodine/kg/day.8.2 Lactation
Risk Summary
There is no information about the presence of ioversol in human or animal milk, the effects of the drug on the breastfed infant, or the effects of the drug on milk production. However, iodinated contrast agents are excreted unchanged in human milk in very low amounts with poor absorption from the gastrointestinal tract of the breastfed infant. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Optiray and any potential adverse effects on the breastfed infant from Optiray or from the underlying maternal condition.Clinical Considerations
Interruption of breastfeeding after exposure to iodinated contrast agents is not necessary because the potential exposure of the breastfed infant to iodine is small. However, a lactating woman may consider interrupting breastfeeding and pumping and discarding breast milk for 8 hours (approximately 5 elimination half-lives) after Optiray administration in order to minimize drug exposure to a breast fed infant.8.4 Pediatric Use
Safety and effectiveness in pediatric patients have been established for the use of Optiray 350 and Optiray 320 in angiocardiography; and for Optiray 320 in contrast enhanced computed tomographic imaging of the head and body, and intravenous excretory urography. Use of Optiray 350 and Optiray 320 in these age groups is based on controlled clinical trials involving 159 patients for pediatric angiocardiography; contrast enhanced computed tomographic imaging of the head and body, and intravenous excretory urography. In general, the types of adverse reactions reported are similar to those of adults [see Adverse Reactions (6.1)].
Safety and effectiveness of Optiray 350 and Optiray 320 have not been established in pediatric patients less than 1 month of age. Safety and effectiveness of Optiray 300 has not been established in pediatric patients.
Pediatric patients at higher risk of experiencing adverse reactions to Optiray include patients with: asthma, sensitivity to medication and/or allergens, congestive heart failure, serum creatinine greater than 1.5 mg/dL, or age less than 12 months. Thyroid function tests indicative of hypothyroidism or transient thyroid suppression have been uncommonly reported following iodinated contrast media administration in pediatric patients, including infants. Some patients were treated for hypothyroidism [See Adverse Reactions (6.2)].
8.5 Geriatric Use
Optiray is substantially excreted by the kidney, and the risk of adverse reactions to Optiray may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, dose selection should be cautious usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy.
-
10 OVERDOSAGE
The adverse effects of overdosage are life-threatening and affect mainly the pulmonary and cardiovascular system. Treatment of an overdosage is directed toward the support of all vital functions and prompt institution of symptomatic therapy.
Ioversol does not bind to plasma or serum protein and is, therefore, dialyzable.
-
11 DESCRIPTION
11.1 Chemical Characteristics
Optiray (ioversol injection) is a non-ionic radiographic contrast agent. Optiray formulations are sterile, nonpyrogenic, aqueous solutions intended for intravascular use. Each bottle is to be used as a Pharmacy Bulk Package for dispensing multiple single dose preparations utilizing a suitable transfer device. Ioversol is designated chemically as N,N'-Bis (2,3-dihydroxypropyl)-5-[N-(2-hydroxyethyl) -glycolamido] -2,4,6-triiodoisophthalamide. The molecular weight of ioversol is 807.11 and the organically bound iodine content is 47.2%.
The structural formula of ioversol is as follows: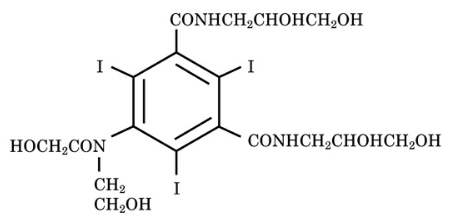
Optiray Pharmacy Bulk Package is available in three strengths:
- Optiray 300 (ioversol injection 64%): Each mL contains 300 mg organically bound iodine, 636 mg ioversol, 3.6 mg, tromethamine, 0.2 mg edetate calcium disodium.
- Optiray 320 (ioversol injection 68%): Each mL contains 320 mg organically bound iodine, 678 mg of ioversol, 3.6 mg tromethamine, 0.2 mg edetate calcium disodium.
- Optiray 350 (ioversol injection 74%): Each mL contains 350 mg organically bound iodine, 741 mg ioversol, 3.6 mg tromethamine, 0.2 mg edetate calcium disodium.
The pH of the Optiray formulations has been adjusted to 6.0 to 7.4 with hydrochloric acid or sodium hydroxide. All solutions are sterilized by autoclaving and contain no preservatives. Ioversol does not dissociate in solution.
11.2 Physical Characteristics
Some physical and chemical properties of these formulations are listed below:
Optiray 300 Optiray 320 Optiray 350 Ioversol content (mg/mL) 636 678 741 Iodine content (mg l/mL) 300 320 350 Osmolality (mOsm/kg water) 651 702 792 Viscosity (cps) at 25°C 8.2 9.9 14.3 at 37°C 5.5 5.8 9.0 Specific Gravity at 37°C 1.352 1.371 1.405 The Optiray formulations are clear, colorless to pale yellow solutions containing no undissolved solids. Crystallization does not occur at room temperature. Optiray solutions have osmolalities 2.3 to 2.8 times that of plasma (285 mOsm/kg water) as shown in the above table and are hypertonic under conditions of use.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Intravascular injection of ioversol opacifies those vessels in the path of the flow of the contrast medium, permitting radiographic visualization of the internal structures until significant hemodilution occurs.
Ioversol may be visualized in the renal parenchyma within 30 to 60 seconds following rapid intravenous injection. Opacification of the calyces and pelves in patients with normal renal function becomes apparent within 1 to 3 minutes, with optimum contrast occurring within 5 to 15 minutes.
Optiray enhances computed tomographic imaging through augmentation of radiographic efficiency. The degree of density enhancement is directly related to the iodine content in an administered dose; peak iodine blood levels occur immediately following rapid intravenous injection. Blood levels fall rapidly within 5 to 10 minutes and the vascular compartment half-life is approximately 20 minutes. This can be accounted for by the dilution in the vascular and extravascular fluid compartments which causes an initial sharp fall in plasma concentration. Equilibration with the extracellular compartments is reached in about 10 minutes; thereafter, the fall becomes exponential.
12.2 Pharmacodynamics
Following administration of Optiray, the degree of enhancement is directly related to the iodine content in an administered dose. Peak iodine plasma levels occur immediately following rapid injection. The time to maximum contrast enhancement can vary, depending on the organ, from the time that peak blood iodine concentrations are reached to one hour after intravenous bolus administration. When a delay between peak blood iodine concentrations and peak contrast is present, it suggests that radiographic contrast enhancement is at least in part dependent on the accumulation of iodine-containing medium within the lesion and outside the blood pool.
For angiography, contrast enhancement is greatest immediately (15 seconds to 120 seconds) after rapid injection. Iodinated contrast agents may be visualized in the renal parenchyma within 30-60 seconds following rapid intravenous injection. Opacification of the calyces and pelves in patients with normal renal function becomes apparent within 1-3 minutes, with optimum contrast occurring within 5-15 minutes.12.3 Pharmacokinetics
Based on the blood clearance curves for 12 healthy volunteers (6 receiving 50 mL and 6 receiving 150 mL of Optiray 320), the biological half-life was 1.5 hours for both doses.
Distribution
In an in vitro human plasma study, ioversol did not bind to protein. The volume of distribution in adults was 0.26 L/kg body weight, consistent with distribution to the extracellular space.Elimination
Metabolism
Ioversol does not undergo significant metabolism, deiodination or biotransformation.Excretion
Greater than 95% of the administered dose was excreted in urine within the first 24 hours, with the peak urine concentration occurring in the first two hours after administration. - 13 NONCLINICAL TOXICOLOGY
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
Optiray is a clear, colorless to pale yellow, sterile, pyrogen-free, aqueous solution available in three strengths. The products are supplied in containers from which the air has been displaced by nitrogen. Optiray is supplied in the following configurations:
NDC Number
Optiray Pharmacy Bulk Package - 350
6x500 mL Pharmacy Bulk Packages 0019-1333-61
Optiray Pharmacy Bulk Package - 320
6x500 mL Pharmacy Bulk Packages 0019-1323-61
Optiray Pharmacy Bulk Package - 300
6x500 mL Pharmacy Bulk Packages 0019-1332-61
-
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions
Advise the patient concerning the risk of hypersensitivity reactions that can occur both during and after Optiray administration. Advise the patient to report any signs or symptoms of hypersensitivity reactions during the procedure and to seek medical attention for signs or symptoms experienced after discharge [see Warnings and Precautions (5.2)].Advise patients to inform their physician if they develop a rash after receiving Optiray [see Warnings and Precautions (5.10)].
Contrast Induced Acute Kidney Injury
Advise the patient concerning appropriate hydration to decrease the risk of contrast induced kidney injury [see Warnings and Precautions (5.3)].Extravasation
If extravasation occurs during injection, advise patients to seek medical care for progression of symptoms [see Warnings and Precautions (5.6)]. -
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Optiray 350 PBP, 500 mL bottle label
For Intravascular Use
Sterile Solution
Optiray™ Pharmacy Bulk Package - 350
500 mL
NDC: 0019-1333-61
IOVERSOL INJECTION 74%
350 mg/mL Organically Bound Iodine
NOT FOR INTRATHECAL USE
Rx only
Pharmacy Bulk Package - Not for Direct Infusion
Protect from light Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Discard contents if product is frozen or if crystallization occurs. Each mL contains 741 mg ioversol, 3.6 mg tromethamine as a buffer and 0.2 mg edetate calcium disodium as a stabilizer. The pH is adjusted with hydrochloric acid or sodium hydroxide.
Usual Dosage: See Package Insert for indications, dosage and dispensing information. Once bottle has been penetrated, withdrawal of contents should be completed without delay. Discard the container no later than 4 hours after initial entry.
12880419Manufactured by:
Liebel-Flarsheim Company LLC
Raleigh, NC 27616
Made in USA
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Optiray 320 PBP, 500 mL bottle label
For Intravascular Use
Sterile Solution
Optiray™ Pharmacy Bulk Package - 320
500 mL
NDC: 0019-1323-61
IOVERSOL INJECTION 68%
320 mg/mL Organically Bound Iodine
NOT FOR INTRATHECAL USE
Rx only
Pharmacy Bulk Package - Not for Direct Infusion
Protect from light Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Discard contents if product is frozen or if crystallization occurs. Each mL contains 678 mg ioversol, 3.6 mg tromethamine as a buffer and 0.2 mg edetate calcium disodium as a stabilizer. The pH is adjusted with hydrochloric acid or sodium hydroxide.
Usual Dosage: See Package Insert for indications, dosage and dispensing information. Once bottle has been penetrated, withdrawal of contents should be completed without delay. Discard the container no later than 4 hours after initial entry.
12840419Manufactured by:
Liebel-Flarsheim Company LLC
Raleigh, NC 27616
Made in USA
-
PACKAGE LABEL - PRINCIPAL DISPLAY PANEL - Optiray 300 PBP, 500 mL bottle label
For Intravascular Use
Sterile Solution
Optiray™ Pharmacy Bulk Package - 300
500 mL
NDC: 0019-1332-61
IOVERSOL INJECTION 64%
300 mg/mL Organically Bound Iodine
NOT FOR INTRATHECAL USE
Rx only
Pharmacy Bulk Package - Not for Direct Infusion
Protect from light Store at 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature]. Discard contents if product is frozen or if crystallization occurs. Each mL contains 636 mg ioversol, 3.6 mg tromethamine as a buffer and 0.2 mg edetate calcium disodium as a stabilizer. The pH is adjusted with hydrochloric acid or sodium hydroxide.
Usual Dosage: See Package Insert for indications, dosage and dispensing information. Once bottle has been penetrated, withdrawal of contents should be completed without delay. Discard the container no later than 4 hours after initial entry.
12910419
Manufactured by:
Liebel-Flarsheim Company LLC
Raleigh, NC 27616
Made in USA
-
INGREDIENTS AND APPEARANCE
OPTIRAY 350
ioversol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0019-1333 Route of Administration INTRA-ARTERIAL, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOVERSOL (UNII: N3RIB7X24K) (IOVERSOL - UNII:N3RIB7X24K) IOVERSOL 741 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) 3.6 mg in 1 mL EDETATE CALCIUM DISODIUM (UNII: 25IH6R4SGF) 0.2 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0019-1333-51 12 in 1 CARTON 10/17/2011 1 250 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 2 NDC: 0019-1333-61 6 in 1 CARTON 10/17/2011 2 500 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020923 10/17/2011 OPTIRAY 320
ioversol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0019-1323 Route of Administration INTRA-ARTERIAL, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOVERSOL (UNII: N3RIB7X24K) (IOVERSOL - UNII:N3RIB7X24K) IOVERSOL 678 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) 3.6 mg in 1 mL EDETATE CALCIUM DISODIUM (UNII: 25IH6R4SGF) 0.2 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0019-1323-61 6 in 1 CARTON 10/17/2011 1 500 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020923 10/17/2011 OPTIRAY 300
ioversol injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0019-1332 Route of Administration INTRA-ARTERIAL, INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IOVERSOL (UNII: N3RIB7X24K) (IOVERSOL - UNII:N3RIB7X24K) IOVERSOL 636 mg in 1 mL Inactive Ingredients Ingredient Name Strength TROMETHAMINE (UNII: 023C2WHX2V) 3.6 mg in 1 mL EDETATE CALCIUM DISODIUM (UNII: 25IH6R4SGF) 0.2 mg in 1 mL HYDROCHLORIC ACID (UNII: QTT17582CB) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0019-1332-61 6 in 1 CARTON 10/17/2011 1 500 mL in 1 BOTTLE, GLASS; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020923 10/17/2011 Labeler - Liebel-Flarsheim Company LLC (057880002) Establishment Name Address ID/FEI Business Operations LIEBEL-FLARSHEIM COMPANY LLC 109024984 ANALYSIS(0019-1333, 0019-1323, 0019-1332) , MANUFACTURE(0019-1333, 0019-1323, 0019-1332) Establishment Name Address ID/FEI Business Operations SpecGx LLC 163205300 API MANUFACTURE(0019-1333, 0019-1323, 0019-1332) Establishment Name Address ID/FEI Business Operations Guerbet Ireland Unlimited Company 988184370 API MANUFACTURE(0019-1333, 0019-1323, 0019-1332)
Trademark Results [Optiray]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 OPTIRAY 73710159 1526467 Live/Registered |
MALLINCKRODT, INC. 1988-02-08 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
