AMIODARONE HYDROCHLORIDE injection, solution
Amiodarone Hydrochloride by
Drug Labeling and Warnings
Amiodarone Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by AuroMedics Pharma LLC, Glenmark Life Sciences Limited, Eugia Pharma Specialities Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AMIODARONE HYDROCHLORIDE INJECTION safely and effectively. See full prescribing information for AMIODARONE HYDROCHLORIDE INJECTION.
AMIODARONE HYDROCHLORIDE injection, for intravenous use
Initial U.S. Approval: 1985INDICATIONS AND USAGE
Amiodarone hydrochloride injection is an antiarrhythmic agent indicated for initiation of treatment and prophylaxis of frequently recurring ventricular fibrillation (VF) and hemodynamically unstable ventricular tachycardia (VT) in patients refractory to other therapy. (1)
DOSAGE AND ADMINISTRATION
- The recommended starting dose is about 1000 mg over the first 24 hours of therapy, delivered by the following infusion regimen (2):
- Initial Load: 150 mg in 100 mL (in D5W) infused over 10 minutes
- Followed by: 1 mg/min for 6 hours
- Followed by: 0.5 mg/min thereafter
- For breakthrough episodes of VF or hemodynamically unstable VT, repeat the Initial Load (2)
DOSAGE FORMS AND STRENGTHS
Injection, 50 mg/mL (3)
CONTRAINDICATIONS
Amiodarone is contraindicated in patients with (4):
- Known hypersensitivity to any of the components of amiodarone, including iodine
- Cardiogenic shock
- Marked sinus bradycardia
- Second- or third-degree atrio-ventricular (AV) block unless a functioning pacemaker is available.
WARNINGS AND PRECAUTIONS
ADVERSE REACTIONS
- The most common adverse reactions (1 to 2%) leading to discontinuation of intravenous amiodarone therapy are hypotension, asystole/cardiac arrest/pulseless electrical activity, VT, and cardiogenic shock. (6)
- Other important adverse reactions are, torsade de pointes, congestive heart failure, and liver function test abnormalities. (6)
To report SUSPECTED ADVERSE REACTIONS, contact AuroMedics Pharma LLC at 1-866-850-2876 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.DRUG INTERACTIONS
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2017
- The recommended starting dose is about 1000 mg over the first 24 hours of therapy, delivered by the following infusion regimen (2):
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Hypotension
5.2 Bradycardia and Atrio-ventricular Block
5.3 Hepatic Injury
5.4 Proarrhythmia
5.5 Pulmonary Injury
5.6 Loss of Vision
5.7 Thyroid Abnormalities
5.8 Neonatal Injury
5.9 Exaggerated Effects of Perisurgical Therapy
5.10 Interference with Corneal Refractive Laser Surgery
5.11 Hypersensitivity Reactions
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Post-Marketing Experience
7 DRUG INTERACTIONS
7.1 Pharmacodynamic Interactions
7.2 Pharmacokinetic Interactions
7.3 Serious Symptomatic Bradycardia When Co-administered with Ledipasvir/Sofosbuvir or with Sofosbuvir with Simeprevir
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Labor and Delivery
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
Amiodarone hydrochloride injection is indicated for initiation of treatment and prophylaxis of frequently recurring ventricular fibrillation (VF) and hemodynamically unstable ventricular tachycardia (VT) in patients refractory to other therapy. Amiodarone also can be used to treat patients with VT/VF for whom oral amiodarone is indicated, but who are unable to take oral medication. During or after treatment with amiodarone, patients may be transferred to oral amiodarone therapy [see Dosage and Administration (2)].
Use amiodarone for acute treatment until the patient's ventricular arrhythmias are stabilized. Most patients will require this therapy for 48 to 96 hours, but amiodarone may be safely administered for longer periods if necessary. -
2 DOSAGE AND ADMINISTRATION
Amiodarone shows considerable interindividual variation in response. Although a starting dose adequate to suppress life-threatening arrhythmias is needed, close monitoring with adjustment of dose is essential. The recommended starting dose of amiodarone is about 1000 mg over the first 24 hours of therapy, delivered by the following infusion regimen:
Table 1: AMIODARONE DOSE RECOMMENDATIONS: FIRST 24 HOURS Loading
infusions
First Rapid:
150 mg over the FIRST 10 minutes (15 mg/min).
Add 3 mL of amiodarone (150 mg) to 100 mL D5W (concentration = 1.5 mg/mL). Infuse 100 mL over 10 minutes.
Followed by
Slow:
360 mg over the NEXT 6 hours (1 mg/min).
Add 18 mL of amiodarone (900 mg) to 500 mL D5W (concentration = 1.8 mg/mL). Infuse 200 mL at a rate of 0.556 mL/min
Maintenance infusion
540 mg over the REMAINING 18 hours (0.5 mg/min).
Decrease the rate of the slow loading infusion to 0.278 mL/min.
After the first 24 hours, continue the maintenance infusion rate of 0.5 mg/min (720 mg per 24 hours) utilizing a concentration of 1 to 6 mg/mL (Use a central venous catheter for amiodarone concentrations greater than 2 mg/mL). The rate of the maintenance infusion may be increased to achieve effective arrhythmia suppression.
In the event of breakthrough episodes of VF or hemodynamically unstable VT, use 150 mg supplemental infusions of amiodarone (mixed in 100 mL of D5W and infused over 10 minutes to minimize the potential for hypotension).
The first 24-hour dose may be individualized for each patient; however, in controlled clinical trials, mean daily doses above 2100 mg were associated with an increased risk of hypotension. Do not exceed an initial infusion rate of 30 mg/min.
Based on the experience from clinical studies of intravenous amiodarone, a maintenance infusion of up to 0.5 mg/min can be continued for 2 to 3 weeks regardless of the patient's age, renal function, or left ventricular function. There has been limited experience in patients receiving intravenous amiodarone for longer than 3 weeks.
The surface properties of solutions containing injectable amiodarone are altered such that the drop size may be reduced. This reduction may lead to underdosage of the patient by up to 30% if drop counter infusion sets are used. Amiodarone must be delivered by a volumetric infusion pump.
Administer amiodarone, whenever possible, through a central venous catheter dedicated to that purpose. Use an in-line filter during administration.
Intravenous amiodarone loading infusions at much higher concentrations and rates of infusion much faster than recommended have resulted in hepatocellular necrosis and acute renal failure, leading to death [see Warnings and Precautions (5.3)].
Intravenous amiodarone concentrations greater than 3 mg/mL in D5W have been associated with a high incidence of peripheral vein phlebitis; however, concentrations of 2.5 mg/mL or less appear to be less irritating. Therefore, for infusions longer than 1 hour, do not exceed amiodarone concentrations of 2 mg/mL, unless a central venous catheter is used [see Adverse Reactions (6.2)].
Amiodarone infusions exceeding 2 hours must be administered in glass or polyolefin bottles containing D5W. Do not use evacuated glass containers for admixing, as incompatibility with a buffer in the container may cause precipitation.
Amiodarone adsorbs to polyvinyl chloride (PVC) tubing, but all of the clinical experience has been with PVC tubing and the concentrations and rates of infusion provided in DOSAGE AND ADMINISTRATION reflect dosing in these studies.
Amiodarone has been found to leach out plasticizers, including DEHP [di-(2-ethylhexyl)phthalate] from intravenous tubing (including PVC tubing). The degree of leaching increases when infusing amiodarone at higher concentrations and lower flow rates than provided in DOSAGE AND ADMINISTRATION. Polysorbate 80, a component of amiodarone hydrochloride injection, is also known to leach DEHP from PVC [see Description (11)].
Amiodarone does not need to be protected from light during administration.
NOTE: Inspect parenteral drug products for particulate matter and discoloration prior to administration, whenever solution and container permit – solution should be clear.
CAUTION: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before the administration of the fluid from the secondary container is complete.
Table 2: AMIODARONE HYDROCHLORIDE SOLUTION STABILITY Solution
Concentration
(mg/mL)
Container
Comments
5% Dextrose in Water (D5W)
1 to 6
PVC
Physically compatible,
with amiodarone loss
<10% at 2 hours at room
temperature.
5% Dextrose in Water (D5W)
1 to 6
Polyolefin, Glass
Physically compatible,
with no amiodarone loss
at 24 hours at room
temperature.
Admixture Incompatibility
Amiodarone in D5W Injection forms precipitates with the drugs shown in Table 3. If co-administration of the following drugs is necessary, use separate intravenous administration lines.
Table 3: Y-SITE INJECTION INCOMPATIBILITY Drug
Vehicle
Amiodarone
Concentration
D5W = Dextrose 5% in Sterile Water, NS = Normal Saline Aminophylline
D5W; NS
4 mg/mL
Amoxicillin Sodium-Clavulanic Acid
unknown
12.5 mg/mL
Ampicillin Sodium-Sulbactam Sodium
NS
6 mg/mL
Argatroban
D5W
1.8 mg/mL
Bivalirudin
D5W
4 mg/mL
Cefamandole Nafate
D5W
4 mg/mL
Cefazolin Sodium
D5W
4 mg/mL
Ceftazidime
D5W
6 mg/mL
Digoxin
D5W
6 mg/mL
Furosemide (10 mg/mL)
D5W
6 mg/mL
Mezlocillin Sodium
D5W
4 mg/mL
Heparin Sodium
D5W
--
Imipenem-Cilastin Sodium
D5W
6 mg/mL
Magnesium Sulfate (500 mg/mL)
D5W
6 mg/mL
Micafungin
NS
4 mg/mL
Piperacillin Sodium –Tazobactam Sodium
D5W
6 mg/mL
Potassium Phosphates
D5W
6 mg/mL
Sodium Bicarbonate
D5W
3 mg/mL
Sodium Nitroprusside
D5W
1.5, 6 and 15 mg/mL
Sodium Phosphates
D5W
6 mg/mL
Intravenous to Oral Transition
Patients whose arrhythmias have been suppressed by amiodarone may be switched to oral amiodarone. The optimal dose for changing from intravenous to oral administration of amiodarone will depend on the dose of intravenous amiodarone already administered, as well as the bioavailability of oral amiodarone. When changing to oral amiodarone therapy, clinical monitoring is recommended, particularly for elderly patients. See package insert for oral amiodarone.
Since grapefruit juice is known to inhibit CYP3A-mediated metabolism of oral amiodarone in the intestinal mucosa, resulting in increased plasma levels of amiodarone, do not drink grapefruit juice during treatment with oral amiodarone [see Drug Interactions (7)].
Table 4 provides suggested doses of oral amiodarone to be initiated after varying durations of amiodarone administration. These recommendations are made on the basis of a similar total body amount of amiodarone delivered by the intravenous and oral routes, based on 50% bioavailability of oral amiodarone.
Table 4: RECOMMENDATIONS FOR ORAL DOSAGE AFTER INTRAVENOUS INFUSION # Assuming a 720 mg/day infusion (0.5 mg/min).
* Intravenous amiodarone is not intended for maintenance treatment.
Duration of Amiodarone Infusion#
Initial Daily Dose of
Oral Amiodarone
< 1 week
800 to 1600 mg
1 to 3 weeks
600 to 800 mg
> 3 weeks*
400 mg
- 3 DOSAGE FORMS AND STRENGTHS
-
4 CONTRAINDICATIONS
Amiodarone is contraindicated in patients with:
- Known hypersensitivity to any of the components of Amiodarone Hydrochloride Injection, including iodine. Hypersensitivity reactions may involve rash, angioedema, cutaneous/mucosal hemorrhage (bleeding), fever, arthralgias (joint pains), eosinophilia (abnormal blood counts), urticaria (hives), thrombotic thrombocytopenic purpura, or severe periarteritis (inflammation around blood vessels).
- Cardiogenic shock.
- Marked sinus bradycardia.
- Second- or third-degree atrio-ventricular (AV) block unless a functioning pacemaker is available.
-
5 WARNINGS AND PRECAUTIONS
Amiodarone should be administered only by physicians who are experienced in the treatment of life-threatening arrhythmias, who are thoroughly familiar with the risks and benefits of amiodarone therapy, and who have access to facilities adequate for monitoring the effectiveness and side effects of treatment.
Because of the long half-life of amiodarone and its metabolite desethylamiodarone, the potential for adverse reactions or interactions, as well as observed adverse effects, can persist following amiodarone withdrawal.5.1 Hypotension
Hypotension is the most common adverse reaction seen with intravenous amiodarone. In clinical trials, treatment-emergent, drug-related hypotension was reported as an adverse effect in 288 (16%) of 1836 patients treated with intravenous amiodarone. Clinically significant hypotension during infusions was seen most often in the first several hours of treatment and was not dose related, but appeared to be related to the rate of infusion. Hypotension necessitating alterations in intravenous amiodarone therapy was reported in 3% of patients, with permanent discontinuation required in less than 2% of patients.
Treat hypotension initially by slowing the infusion; additional standard therapy may be needed, including the following: vasopressor drugs, positive inotropic agents, and volume expansion. Monitor the initial rate of infusion closely and do not exceed the recommended rate [see Dosage and Administration (2)].
In some cases, hypotension may be refractory and result in a fatal outcome [see Adverse Reactions (6.2)].5.2 Bradycardia and Atrio-ventricular Block
In 90 (4.9%) of 1836 patients in clinical trials, drug-related bradycardia that was not dose-related occurred while they were receiving intravenous amiodarone for life-threatening VT/VF. Treat bradycardia by slowing the infusion rate or discontinuing amiodarone. In some patients, a pacemaker is required. Despite such measures, bradycardia was progressive and terminal in 1 patient during the controlled trials. Treat patients with a known predisposition to bradycardia or AV block with amiodarone in a setting where a temporary pacemaker is available.
5.3 Hepatic Injury
Elevations of blood hepatic enzyme values [alanine aminotransferase (ALT), aspartate aminotransferase (AST), and gamma-glutamyl transferase (GGT)] are commonly seen in patients with immediately life-threatening VT/VF. Interpreting elevated AST activity can be difficult because the values may be elevated in patients who have had recent myocardial infarction, congestive heart failure, or multiple electrical defibrillations. Approximately 54% of patients receiving intravenous amiodarone in clinical studies had baseline liver enzyme elevations, and 13% had clinically significant elevations. In 81% of patients with both baseline and on-therapy data available, the liver enzyme elevations either improved during therapy or remained at baseline levels. Baseline abnormalities in hepatic enzymes are not a contraindication to treatment. Elevated bilirubin levels have been reported in patients administered intravenous amiodarone.
Acute, centrolobular confluent hepatocellular necrosis leading to hepatic coma, acute renal failure, and death has been associated with the administration of intravenous amiodarone (see Dosage and Administration (2)).
In patients with life-threatening arrhythmias, the potential risk of hepatic injury should be weighed against the potential benefit of amiodarone therapy. Carefully monitor patients receiving amiodarone for evidence of progressive hepatic injury. In such cases, consider reducing the rate of administration or withdrawing amiodarone.5.4 Proarrhythmia
Like all antiarrhythmic agents, amiodarone may cause a worsening of existing arrhythmias or precipitate a new arrhythmia sometimes leading to fatal outcomes [see Adverse Reactions (6.2)]. Proarrhythmia, primarily torsade de pointes (TdP), has been associated with prolongation, by intravenous amiodarone, of the QTc interval to 500 ms or greater. Although QTc prolongation occurred frequently in patients receiving intravenous amiodarone, TdP or new-onset VF occurred infrequently (less than 2%). Monitor patients for QTc prolongation during infusion with amiodarone. Reserve the combination of amiodarone with other antiarrhythmic therapies that prolong the QTc to patients with life-threatening ventricular arrhythmias who are incompletely responsive to a single agent.
Correct hypokalemia, hypomagnesemia or hypocalcemia whenever possible before initiating treatment with amiodarone, as these disorders can exaggerate the degree of QTc prolongation and increase the potential for TdP. Give special attention to electrolyte and acid-base balance in patients experiencing severe or prolonged diarrhea or in patients receiving concomitant diuretics and laxatives.
Amiodarone causes thyroid dysfunction in some patients, which may lead to potentially fatal breakthrough or exacerbated arrhythmias.5.5 Pulmonary Injury
Early-onset Pulmonary Toxicity
There have been postmarketing reports of acute-onset (days to weeks) pulmonary injury in patients treated with intravenous amiodarone. Findings have included pulmonary infiltrates and masses on X-ray, bronchospasm, wheezing, fever, dyspnea, cough, hemoptysis, and hypoxia. Some cases have progressed to respiratory failure or death.
ARDS
Two percent (2%) of patients were reported to have adult respiratory distress syndrome (ARDS) during clinical studies involving 48 hours of therapy.
Pulmonary Fibrosis
There have been reports of early development of pulmonary fibrosis (within 1 to 3 months) following initiation of amiodarone treatment. Only 1 of more than 1000 patients treated with intravenous amiodarone in clinical studies developed pulmonary fibrosis. In that patient, the condition was diagnosed 3 months after treatment with intravenous amiodarone, during which time the patient received oral amiodarone. Pulmonary toxicity is a well-recognized complication of long-term amiodarone use (see package insert for oral amiodarone).5.6 Loss of Vision
Cases of optic neuropathy and optic neuritis, usually resulting in visual impairment, have been reported in patients treated with oral amiodarone or intravenous amiodarone. In some cases, visual impairment has progressed to permanent blindness. Optic neuropathy and neuritis may occur at any time following initiation of therapy. A causal relationship to the drug has not been clearly established. Perform an ophthalmic examination if symptoms of visual impairment appear, such as changes in visual acuity and decreases in peripheral vision. Re-evaluate the necessity of amiodarone therapy if optic neuropathy or neuritis is suspected. Perform regular ophthalmic examination, including fundoscopy and slit-lamp examination, during administration of amiodarone.
5.7 Thyroid Abnormalities
Amiodarone inhibits peripheral conversion of thyroxine (T4) to triiodothyronine (T3) and may cause increased T4 levels, decreased T3 levels, and increased levels of inactive reverse T3 (rT3) in clinically euthyroid patients. Amiodarone is also a potential source of large amounts of inorganic iodine and can cause either hypothyroidism or hyperthyroidism. Evaluate thyroid function prior to treatment and periodically thereafter, particularly in elderly patients, and in any patient with a history of thyroid nodules, goiter, or other thyroid dysfunction. Because of the slow elimination of amiodarone and its metabolites, high plasma iodide levels, altered thyroid function, and abnormal thyroid-function tests may persist for months following amiodarone withdrawal.
There have been postmarketing reports of thyroid nodules/thyroid cancer in patients treated with amiodarone. In some instances hyperthyroidism was also present.
Hyperthyroidism and Thyrotoxicosis
Amiodarone causes hyperthyroidism in about 2% of patients. Thyrotoxicosis and arrhythmia with fatal outcome has been reported in the presence of pre-existing hyperthyroidism even following a single intravenous amiodarone dose. Consider the possibility of hyperthyroidism if any new signs of arrhythmia appear.
Hyperthyroidism may result from iodine load (type 1 amiodarone-induced thyrotoxicosis [type 1 AIT]; in particular in patients with underlying autonomous thyroid nodules or latent Grave’s disease). Hyperthyroidism may also result from direct amiodarone-induced destructive thyroiditis that occurs in individuals with no underlying thyroid disease (type 2 AIT), resulting in the release of preformed thyroid hormone into the bloodstream from damaged thyroid follicular epithelium. Mixed forms of hyperthyroidism as a result of both pathogenic mechanisms (excessive thyroid hormone production and thyroid destruction) can also occur. The risk of hyperthyroidism may be higher among patients with prior inadequate dietary iodine intake.
Identify hyperthyroidism by relevant clinical signs and symptoms, subnormal serum levels of thyroid stimulating hormone (TSH), abnormally elevated serum free T4, and elevated or normal serum T3. Since arrhythmia breakthroughs may accompany amiodarone-induced hyperthyroidism, aggressive medical treatment is indicated, including, if possible, dose reduction or withdrawal of amiodarone. Amiodarone hyperthyroidism may be followed by a transient period of hypothyroidism.
The institution of antithyroid drugs, β-adrenergic blockers or temporary corticosteroid therapy may be necessary. The action of antithyroid drugs may be especially delayed in amiodarone-induced thyrotoxicosis because of substantial quantities of preformed thyroid hormones stored in the gland. Radioactive iodine therapy is not recommended because of the low radioiodine uptake associated with amiodarone-induced hyperthyroidism.
When aggressive treatment of amiodarone-induced thyrotoxicosis has failed or amiodarone cannot be discontinued because it is the only drug effective against the resistant arrhythmia, surgical management may be an option. Experience with thyroidectomy as a treatment for amiodarone-induced thyrotoxicosis is limited, and this form of therapy could induce thyroid storm. Therefore, surgical and anesthetic management require careful planning.
Hypothyroidism
Hypothyroidism has been reported in 2 to 10% of patients receiving amiodarone and may be primary or subsequent to resolution of preceding amiodarone-induced hyperthyroidism. This condition may be identified by clinical symptoms and elevated serum TSH levels. Cases of severe hypothyroidism and myxedema coma, sometimes fatal, have been reported in association with amiodarone therapy. In some clinically hypothyroid amiodarone-treated patients, free thyroxine index values may be normal. Manage hypothyroidism by reducing the dose of or discontinuing amiodarone and considering the need for thyroid hormone supplement.5.8 Neonatal Injury
Amiodarone can cause fetal harm when administered to a pregnant woman. Fetal exposure may increase the potential for adverse experiences including cardiac, thyroid, neurodevelopmental, neurological and growth effects in neonates. Inform the patient of the potential hazard to the fetus if amiodarone is administered during pregnancy or if the patient becomes pregnant while taking amiodarone [see Pregnancy (8.1)].
5.9 Exaggerated Effects of Perisurgical Therapy
Perform close perioperative monitoring in patients undergoing general anesthesia who are on amiodarone therapy as they may be more sensitive to the myocardial depressant and conduction defects of halogenated inhalational anesthetics.
-
6 ADVERSE REACTIONS
The following adverse reactions are described elsewhere in labeling:
- Hypotension [see Warnings and Precautions (5.1)]
- Hepatic injury [see Warnings and Precautions (5.3)]
- Rhythm disturbances [see Warnings and Precautions (5.4)]
- Pulmonary injury [see Warnings and Precautions (5.5)]
- Thyroid injury [see Warnings and Precautions (5.7)]
- Hypersensitivity [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
In a total of 1836 patients in controlled and uncontrolled clinical trials, 14% of patients received intravenous amiodarone for at least one week, 5% received it for at least 2 weeks, 2% received it for at least 3 weeks, and 1% received it for more than 3 weeks, without an increased incidence of severe adverse reactions. The mean duration of therapy in these studies was 5.6 days; median exposure was 3.7 days.
The most important adverse reactions were hypotension, asystole/cardiac arrest/pulseless electrical activity (PEA), cardiogenic shock, congestive heart failure, bradycardia, liver function test abnormalities, VT, and AV block. Overall, treatment was discontinued for about 9% of the patients because of adverse reactions. The most common adverse reactions leading to discontinuation of intravenous amiodarone therapy were hypotension (1.6%), asystole/cardiac arrest/PEA (1.2%), VT (1.1%), and cardiogenic shock (1%).
Table 5 lists the most common (incidence ≥2%) adverse reactions during intravenous amiodarone therapy considered at least possibly drug-related. These data were collected in clinical trials involving 1836 patients with life-threatening VT/VF. Data from all assigned treatment groups are pooled because none of the adverse reactions appeared to be dose-related.
Table 5: ADVERSE REACTIONS IN PATIENTS RECEIVING INTRAVENOUS AMIODARONE IN CONTROLLED AND OPEN-LABEL STUDIES (≥ 2% INCIDENCE) Study Event Controlled
Studies
(n = 814)Open-Label
Studies
(n = 1022)Total
(n = 1836)Body as a whole
Fever
24 (2.9%)
13 (1.2%)
37 (2%)
Cardiovascular System
Bradycardia
49 (6%)
41 (4%)
90 (4.9%)
Congestive heart failure
18 (2.2%)
21 (2%)
39 (2.1%)
Heart arrest
29 (3.5%)
26 (2.5%)
55 (2.9%)
Hypotension
165 (20.2%)
123 (12%)
288 (15.6%)
Ventricular tachycardia
15 (1.8%)
30 (2.9%)
45 (2.4%)
Digestive System
Liver function tests abnormal
35 (4.2%)
29 (2.8%)
64 (3.4%)
Nausea
29 (3.5%)
43 (4.2%)
72 (3.9%)
Other adverse reactions reported in less than 2% of patients receiving intravenous amiodarone in controlled and uncontrolled studies included the following: abnormal kidney function, atrial fibrillation, diarrhea, increased ALT, increased AST, lung edema, nodal arrhythmia, prolonged QT interval, respiratory disorder, shock, sinus bradycardia, Stevens-Johnson syndrome, thrombocytopenia, VF, and vomiting.
6.2 Post-Marketing Experience
The following adverse reactions have been reported in the post-marketing experience during or in close temporal relationship to intravenous amiodarone administration. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and Lymphatic System Disorders: pancytopenia, neutropenia, hemolytic anemia, aplastic anemia, thrombocytopenia, and agranulocytosis.
Cardiac Disorders: sinus node dysfunction (sinus arrest, sinoatrial block), intraventricular conduction disorders including bundle branch block and infra-HIS block, bradycardia (sometimes fatal), ventricular extrasystoles, and antegrade conduction via an accessory pathway.
Endocrine Disorders: syndrome of inappropriate antidiuretic hormone secretion (SIADH).
Eye Disorders: visual field defect and blurred vision.
Gastrointestinal Disorders: pancreatitis.
General Disorders and Administration Site Conditions: infusion site reactions, including thrombosis, phlebitis, thrombophlebitis, cellulitis, pain, induration, edema, inflammation, urticaria, pruritus, erythema, pigment changes, hypoesthesia, skin sloughing, extravasation possibly leading to venous/infusion site necrosis, intravascular amiodarone deposition/mass (developed in the superior vena cava around a central venous catheter after long-term [28 days] amiodarone therapy administered through a central line), and granuloma.
Hepatobiliary Disorders: cholestasis, cirrhosis, jaundice, alkaline phosphatase and blood lactate dehydrogenase increase.
Musculoskeletal and Connective Tissue Disorders: myopathy, muscle weakness, rhabdomyolysis, muscle spasms, and back pain.
Neoplasms Benign, Malignant and Unspecified (incl Cysts and Polyps) Disorders: thyroid nodules/thyroid cancer.
Nervous System Disorders: intracranial pressure increased, pseudotumor cerebri, tremor, dizziness and hypoesthesia.
Psychiatric Disorders: confusional state, hallucination, disorientation, and delirium.Renal and Urinary Disorders: acute renal failure (sometimes fatal), renal impairment, renal insufficiency, and blood creatinine increased.
Reproductive Disorders and Breast Disorders: Epididymitis
Respiratory, Thoracic and Mediastinal Disorders: interstitial pneumonitis, bronchiolitis obliterans organizing pneumonia (possibly fatal), pulmonary alveolar hemorrhage, pulmonary phospholipidoisis, pleural effusion, bronchospasm, dyspnea, cough, hemoptysis, wheezing, and hypoxia.
Skin and Subcutaneous Tissue Disorders: toxic epidermal necrolysis (sometimes fatal), Stevens-Johnson syndrome, exfoliative dermatitis, erythema multiforme, skin cancer, pruritus, angioedema, and urticaria.
Vascular Disorders: vasculitis and flushing. -
7 DRUG INTERACTIONS
7.1 Pharmacodynamic Interactions
Drugs prolonging the QT interval: Co-administration of drugs prolonging the QT interval (such as class I and III antiarrhythmics, lithium, certain phenothiazines, tricyclic antidepressants, certain fluoroquinolone and macrolide antibiotics, azole antifungals, halogenated inhalation anesthetic agents) increases the risk of Torsade de Pointes. In general, avoid concomitant use of drugs that prolong the QT interval [see Warnings and Precautions (5.4)].
Drugs that slow heart rate: Concomitant use of drugs with depressant effects on the sinus and AV nodes (e.g., digoxin, beta blockers, verapamil, diltiazem, ivabradine, clonidine) can potentiate the electrophysiologic and hemodynamic effects of amiodarone, resulting in bradycardia, sinus arrest, and AV block. Monitor heart rate in patients on amiodarone and concomitant drugs that slow heart rate.7.2 Pharmacokinetic Interactions
Effect of other drugs on amiodarone
Amiodarone is metabolized to the active metabolite desethylamiodarone (DEA) by the cytochrome P450 (CYP450) enzyme group, specifically CYP3A and CYP2C8.
Amiodarone has the potential for interactions with drugs or substances that may be substrates, inhibitors or inducers of CYP450 enzymes (e.g., inhibitors such as protease inhibitors, grapefruit juice, certain fluoroquinolone and macrolide antibiotics, azole antifungals and inducers such as St. John’s Wort) or P-glycoprotein. In view of the long and variable half- life of amiodarone, potential for drug interactions exists not only with concomitant medications but also with drugs administered after discontinuation of amiodarone [see Clinical Pharmacology (12.3)].
Patients should avoid grapefruit juice beverages while taking amiodarone because exposure to amiodarone is significantly increased [see Clinical Pharmacology (12.3)].
Effect of amiodarone on other drugs
Amiodarone and DEA are inhibitors of P-glycoprotein and certain CYP450 enzymes, including CYP1A2, CYP2C9, CYP2D6, and CYP3A [see Clinical Pharmacology (12.3)].
Antiarrhythmics: The metabolism of quinidine, procainamide, and flecainide can be inhibited by amiodarone. In general, initiate any added antiarrhythmic drug at a lower than usual dose and monitor the patient carefully.
During transfer to oral amiodarone, reduce the dose levels of previously administered antiarrhythmic agents by 30 to 50% several days after the addition of oral amiodarone. Review the continued need for the other antiarrhythmic agent after the effects of amiodarone have been established, and attempt discontinuation [see Clinical Pharmacology (12.3)].
Digoxin: In patients receiving digoxin therapy, administration of oral amiodarone results in an increase in serum digoxin concentration. Reduce dose of digoxin by half or discontinue digoxin. If digitalis treatment is continued, monitor serum levels closely and observe patients for clinical evidence of toxicity [see Clinical Pharmacology (12.3)].
HMG-CoA Reductase Inhibitors:
Limit the dose of simvastatin in patients on amiodarone to 20 mg daily. Limit the daily dose of lovastatin to 40 mg. Lower starting and maintenance doses of other CYP3A4 substrates (e.g., atorvastatin) may be required as amiodarone may increase the plasma concentration of these drugs.
Anticoagulants: Potentiation of warfarin-type (CYP2C9 and CYP3A substrate) anticoagulant response is almost always seen in patients receiving amiodarone and can result in serious or fatal bleeding. Since the concomitant administration of warfarin with amiodarone increases INR by 100% after 3 to 4 days, reduce the dose of the anticoagulant by one-third to one-half, and monitor INR closely.
Cyclosporine (CYP3A substrate) administered in combination with oral amiodarone has been reported to produce persistently elevated plasma concentrations of cyclosporine resulting in elevated creatinine, despite reduction in dose of cyclosporine. Monitor cyclosporine drug levels and renal function in patients taking both drugs.
Increased steady-state levels of phenytoin during concomitant therapy with amiodarone have been reported. Monitor phenytoin levels in patients taking both drugs.7.3 Serious Symptomatic Bradycardia When Co-administered with Ledipasvir/Sofosbuvir or with Sofosbuvir with Simeprevir
Postmarketing cases of symptomatic bradycardia, some requiring pacemaker insertion and at least one fatal, have been reported when ledipasvir/sofosbuvir or sofosbuvir with simeprevir were initiated in patients on amiodarone. Bradycardia generally occurred within hours to days, but in some cases up to 2 weeks after initiating antiviral treatment. Bradycardia generally resolved after discontinuation of antiviral treatment. The mechanism for this effect is unknown. Monitor heart rate in patients taking or recently discontinuing amiodarone when starting antiviral treatment.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Category D [see Warnings and Precautions (5.8)].
Teratogenic Effects
Amiodarone and desethylamiodarone cross the placenta.
Reported risks include:
- neonatal bradycardia, QT prolongation, and periodic ventricular extrasystoles
- neonatal hypothyroidism(with or without goiter) detected antenatally or in the newborn and reported even after a few days of exposure
- neonatal hyperthyroxinemia
- neurodevelopmental abnormalities independent of thyroid function, including speech delay and difficulties with written language and arithmetic, delayed motor development, and ataxia.
- jerk nystagmus with synchronous head titubation
- fetal growth retardation
- premature birth
Amiodarone has caused a variety of adverse effects in animals.
Amiodarone was given intravenously to rabbits at dosages of 5, 10, or 25 mg/kg per day (about 0.1, 0.3, and 0.7 times the human intravenous maintenance dose of 0.5 mg/min on a body surface area basis), during gestation days 8 to 16 (organogenesis). The incidence of maternal deaths increased with increasing dose and occurred in all treated groups, and controls. Mean fetal weights were significantly decreased in the low and middle dose groups and embryotoxicity (as manifested by fewer full- term fetuses and increased resorptions) occurred at dosages of 10 mg/kg and above. There were no significant differences in the number of minor fetal abnormalities and no major fetal abnormalities were observed.
Amiodarone was administered by continuous intravenous infusion to rats at dosages of 25, 50, or 100 mg/kg per day (about 0.3, 0.7, and 1.3 times the human intravenous maintenance dose of 0.5 mg/min on a body surface area basis) during gestation days 8 to 16 (organogenesis). Maternal toxicity (manifest as reduced weight gain and food consumption) and embryotoxicity (manifest as increased resorptions, decreased live litter size and fetal body weights, and delayed sternal and metacarpal ossification) were observed in the 100 mg/kg group. The delayed ossification was reversible and related to decreased fetal weight. Fetal thyroid tissues appeared normal in all groups.
Nonteratogenic Effects
Very high concentrations of amiodarone and desethylamiodarone may be found in testes. Elevated follicle-stimulating hormone and luteinizing hormone levels, suggestive of testicular dysfunction, have been reported in men on long-termamiodarone treatment.
While planning pregnancy after discontinuation of amiodarone treatment, consider the long half-life of amiodarone and its metabolite DEA.
8.2 Labor and Delivery
It is not known whether the use of amiodarone during labor or delivery has any immediate or delayed adverse effects. Preclinical studies in rodents have not shown any effect on the duration of gestation or on parturition.
8.3 Nursing Mothers
Amiodarone and one of its major metabolites, desethylamiodarone (DEA), are excreted in human milk, suggesting that breast-feeding could expose the nursing infant to a significant dose of the drug. Nursing offspring of lactating rats administered amiodarone have demonstrated reduced viability and reduced body weight gains. The risk of exposing the infant to amiodarone must be weighed against the potential benefit of arrhythmia suppression in the mother. Advise the mother to discontinue nursing.
8.4 Pediatric Use
The safety and effectiveness of amiodarone in pediatric patients have not been established; therefore, the use of amiodarone in pediatric patients is not recommended. In a pediatric trial of 61 patients, aged 30 days to 15 years, hypotension (36%), bradycardia (20%), and AV block (15%) were common dose-related adverse reactions and were severe or life-threatening in some cases. Injection site reactions were seen in 5 (25%) of the 20 patients receiving intravenous amiodarone through a peripheral vein irrespective of dose regimen.
Amiodarone hydrochloride injection contains the preservative benzyl alcohol [see Description (11)]. There have been reports of fatal “gasping syndrome” in neonates (children less than one month of age) following the administration of intravenous solutions containing the preservative benzyl alcohol. Symptoms include a striking onset of gasping respiration, hypotension, bradycardia, and cardiovascular collapse.8.5 Geriatric Use
Clinical studies of amiodarone did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Carefully consider dose selection in an elderly patient. In general, start at the low end of the dosing range in the elderly to reflect the greater frequency of decreased hepatic, renal, or cardiac function, and concomitant disease or other drug therapy.
-
10 OVERDOSAGE
There have been cases, some fatal, of amiodarone overdose. Effects of an inadvertent overdose of intravenous amiodarone include hypotension, cardiogenic shock, bradycardia, AV block, and hepatotoxicity. Treat hypotension and cardiogenic shock by slowing the infusion rate or with standard therapy: vasopressor drugs, positive inotropic agents, and volume expansion. Bradycardia and AV block may require temporary pacing. Monitor hepatic enzyme concentrations closely. Neither amiodarone nor DEA is dialyzable.
-
11 DESCRIPTION
Amiodarone hydrochloride injection, USP contains amiodarone hydrochloride (C25H29I2NO3HCl), a class III antiarrhythmic drug. Amiodarone hydrochloride is (2-butyl-3-benzo-furanyl)[4-[2-(diethylamino)ethoxy]-3,5-diiodophenyl]methanone hydrochloride.
Amiodarone hydrochloride has the following structural formula:
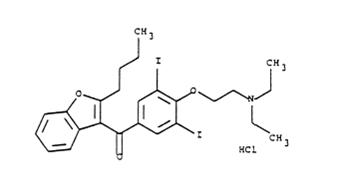
Amiodarone hydrochloride USP is a white or almost white, fine crystalline powder, and is very slightly soluble in water. It has a molecular weight of 681.78 and contains 37.3% iodine by weight. Amiodarone hydrochloride injection, USP is a sterile clear, pale yellow solution visually free from particulates. Each milliliter of the amiodarone formulation contains 50 mg of amiodarone hydrochloride USP, 20.2 mg of benzyl alcohol, 100 mg of polysorbate 80, and water for injection.
Amiodarone hydrochloride injection, USP contains polysorbate 80, which is known to leach di-(2-ethylhexyl)phthalate (DEHP) from polyvinylchloride (PVC) [(see Dosage and Administration (2)]. -
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Amiodarone is generally considered a class III antiarrhythmic drug, but it possesses electrophysiologic characteristics of all four Vaughan Williams classes. Like class I drugs, amiodarone blocks sodium channels at rapid pacing frequencies, and like class II drugs, amiodarone exerts a noncompetitive antisympathetic action. One of its main effects, with prolonged administration, is to lengthen the cardiac action potential, a class III effect. The negative chronotropic effect of amiodarone in nodal tissues is similar to the effect of class IV drugs. In addition to blocking sodium channels, amiodarone blocks myocardial potassium channels, which contributes to slowing of conduction and prolongation of refractoriness. The antisympathetic action and the block of calcium and potassium channels are responsible for the negative dromotropic effects on the sinus node and for the slowing of conduction and prolongation of refractoriness in the atrioventricular (AV) node. Its vasodilatory action can decrease cardiac workload and consequently myocardial oxygen consumption.
Intravenous amiodarone administration prolongs intranodal conduction (Atrial-His, AH) and refractoriness of the atrioventricular node (ERP AVN), but has little or no effect on sinus cycle length (SCL), refractoriness of the right atrium and right ventricle (ERP RA and ERP RV), repolarization (QTc), intraventricular conduction (QRS), and infra-nodal conduction (His-ventricular, HV). A comparison of the electrophysiologic effects of intravenous amiodarone and oral amiodarone is shown in the table below.
Table 6: EFFECTS OF INTRAVENOUS AND ORAL AMIODARONE ON ELECTROPHYSIOLOGIC PARAMETERS Formulation
SCL
QRS
QTc
AH
HV
ERP
RA
ERP
RV
ERP
AVN
Intravenous
↔
↔
↔
↑
↔
↔
↔
↑
Oral
↑
↔
↑
↑
↔
↑
↑
↑
↔ No change
At higher doses (>10 mg/kg) of intravenous amiodarone, prolongation of the ERP RV and modest prolongation of the QRS have been seen. These differences between oral and IV administration suggest that the initial acute effects of intravenous amiodarone may be predominately focused on the AV node, causing an intranodal conduction delay and increased nodal refractoriness due to slow channel blockade (class IV activity) and noncompetitive adrenergic antagonism (class II activity).12.2 Pharmacodynamics
Intravenous amiodarone has been reported to produce negative inotropic and vasodilatory effects in animals and humans. In clinical studies of patients with refractory VF or hemodynamically unstable VT, treatment-emergent, drug-related hypotension occurred in 288 of 1836 patients (16%) treated with intravenous amiodarone. No correlations were seen between the baseline ejection fraction and the occurrence of clinically significant hypotension during infusion of intravenous amiodarone.
No data are available on the activity of DEA in humans, but in animals, it has significant electrophysiologic and antiarrhythmic effects generally similar to amiodarone itself. DEA's precise role and contribution to the antiarrhythmic activity of oral amiodarone are not certain. The development of maximal ventricular class III effects after oral amiodarone administration in humans correlates more closely with DEA accumulation over time than with amiodarone accumulation. On the other hand, after intravenous amiodarone administration, there is evidence of activity well before significant concentrations of DEA are attained [see Clinical Trials (14)].12.3 Pharmacokinetics
Disposition:
Amiodarone exhibits complex disposition characteristics after intravenous administration. Peak serum concentrations after single 5 mg/kg 15-minute intravenous infusions in healthy subjects range between 5 and 41 mg/L. Peak concentrations after 10-minute infusions of 150 mg intravenous amiodarone in patients with ventricular fibrillation (VF) or hemodynamically unstable ventricular tachycardia (VT) range between 7 and 26 mg/L. Due to rapid distribution, serum concentrations decline to 10% of peak values within 30 to 45 minutes after the end of the infusion. In clinical trials, after 48 hours of continued infusions (125, 500 or 1000 mg/day) plus supplemental (150 mg) infusions (for recurrent arrhythmias), amiodarone mean serum concentrations between 0.7 to 1.4 mg/L were observed (n=260).
Metabolism:
N-desethylamiodarone (DEA) is the major active metabolite of amiodarone in humans. DEA serum concentrations above 0.05 mg/L are not usually seen until after several days of continuous infusion but with prolonged therapy reach approximately the same concentration as amiodarone. Amiodarone is metabolized to DEA by the cytochrome P450 (CYP450) enzyme group, specifically cytochrome CYP3A and CYP2C8. The CYP3A isoenzyme is present in both the liver and intestines. The highly variable systemic availability of oral amiodarone may be attributed to large interindividual variability in CYP3A activity.
Distribution/Elimination:
From in vitro studies, the protein binding of amiodarone is >96%. Amiodarone and DEA cross the placenta and both appear in breast milk. Neither amiodarone nor DEA is dialyzable.
Amiodarone is eliminated primarily by hepatic metabolism and biliary excretion and there is negligible excretion of amiodarone or DEA in urine. In studies in healthy subjects following single intravenous administration (5 mg/kg of amiodarone over 15 min), the plasma concentration vs. time profile could be characterized by linear sum of four exponential terms with terminal elimination half-lives (t½) of 9 to 36 days for amiodarone and 9 to 30 days for DEA. The clearance of amiodarone and DEA ranged between 63 to 231 mL/hr/kg and 140 to 400 mL/h/kg, respectively. In clinical studies of 2 to 7 days, clearance of amiodarone after intravenous administration in patients with VT and VF ranged between 220 and 440 mL/hr/kg.
Special Populations:
Effect of Age: The pharmacokinetics of amiodarone and DEA are affected by age. Normal subjects over 65 years of age show lower clearances (about 100 mL/hr/kg) than younger subjects (about 150 mL/hr/kg) and an increase in t½ from about 20 to 47 days.
Effect of Gender: Pharmacokinetics of amiodarone and DEA are similar in males and females.
Renal Impairment: Renal disease does not influence the pharmacokinetics of amiodarone or DEA.
Hepatic Impairment: After a single dose of intravenous amiodarone to cirrhotic patients, significantly lower Cmax and average concentration values are seen for DEA, but mean amiodarone levels are unchanged.
Cardiac Disease: In patients with severe left ventricular dysfunction, the pharmacokinetics of amiodarone are not significantly altered but the terminal elimination t½ of DEA is prolonged.
Although no dosage adjustment for patients with renal, hepatic, or cardiac abnormalities has been defined during chronic treatment with oral amiodarone, close clinical monitoring is prudent for elderly patients and those with severe left ventricular dysfunction.
Exposure-Response: There is no established relationship between drug concentration and therapeutic response for short-term intravenous use.
Drug Interactions:
Effect of other drugs on amiodarone:
Cimetidine inhibits CYP3A and can increase serum amiodarone levels.
Grapefruit juice given to healthy volunteers increased amiodarone AUC by 50% and Cmax by 84%, resulting in increased plasma levels of amiodarone.
Cholestyramine reduces enterohepatic circulation of amiodarone thereby increasing its elimination. This results in reduced amiodarone serum levels and half-life.
Effect of amiodarone on other drugs:
Amiodarone taken concomitantly with quinidine increases the quinidine serum concentration by 33% after two days. Amiodarone taken concomitantly with procainamide for less than seven days increases plasma concentrations of procainamide and n-acetyl procainamide by 55% and 33%, respectively.
Loratadine, a non-sedating antihistaminic, is metabolized primarily by CYP3A and its metabolism can be inhibited by amiodarone.
Metabolism of lidocaine can be inhibited by amiodarone. Sinus bradycardia has been reported with oral amiodarone in combination with lidocaine (CYP3A substrate) given for local anesthesia. Seizure, associated with increased lidocaine concentrations, has been reported with concomitant administration of intravenous amiodarone.
Amiodarone can inhibit the metabolism of macrolide/ketolide antibiotics (except for azithromycin) and systemic azole antifungal drugs.
Amiodarone taken concomitantly with digoxin increases the serum digoxin concentration by 70% after one day.
Dextromethorphan is a substrate for both CYP2D6 and CYP3A. Amiodarone inhibits CYP2D6. Chronic (> 2 weeks) oral amiodarone administration impairs metabolism of dextromethorphan can lead to increased serum concentrations.
Dabigatran etexilate when taken concomitantly with oral amiodarone can result in elevated serum concentration of dabigatran.
Cyclophosphamide is a prodrug, metabolized by CYP450 including CYP3A to an active metabolite. The metabolismof cyclophosphamide may be inhibited by amiodarone.
Clopidogrel, an inactive thienopyridine prodrug, is metabolized in the liver by CYP3A to an active metabolite. A potential interaction between clopidogrel and amiodarone resulting in ineffective inhibition of platelet aggregation has been reported. -
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity studies were conducted with intravenous administration of amiodarone. However, oral amiodarone caused a statistically significant, dose-related increase in the incidence of thyroid tumors (follicular adenoma and carcinoma) in rats. The incidence of thyroid tumors in rats was greater than the incidence in controls even at the lowest dose level tested, i.e., 5 mg/kg/day (much less, on a body surface area basis, than the maximum recommended human maintenance dose of 600 mg/day).
Mutagenicity studies conducted with amiodarone hydrochloride (Ames, micronucleus, and lysogenic induction tests) were negative.
No fertility studies were conducted with intravenous administration of amiodarone. However, in a study in which amiodarone hydrochloride was orally administered to male and female rats, beginning 9 weeks prior to mating, reduced fertility was observed at a dose level of 90 mg/kg/day (approximately 1.4 times the maximum recommended human maintenance dose of 600 mg/day). -
14 CLINICAL STUDIES
Apart from studies in patients with VT or VF, described below, there are two other studies of amiodarone showing an antiarrhythmic effect before significant levels of DEA could have accumulated. A placebo-controlled study of intravenous amiodarone (300 mg over 2 hours followed by 1200 mg/day) in post-coronary artery bypass graft patients with supraventricular and 2- to 3-consecutive-beat ventricular arrhythmias showed a reduction in arrhythmias from 12 hours on. A baseline-controlled study using a similar IV regimen in patients with recurrent, refractory VT/VF also showed rapid onset of antiarrhythmic activity; amiodarone therapy reduced episodes of VT by 85% compared to baseline.
The acute effectiveness of intravenous amiodarone in suppressing recurrent VF or hemodynamically unstable VT is supported by two randomized, parallel, dose-response studies of approximately 300 patients each. In these studies, patients with at least two episodes of VF or hemodynamically unstable VT in the preceding 24 hours were randomly assigned to receive doses of approximately 125 or 1000 mg over the first 24 hours, an 8-fold difference. In one study, a middle dose of approximately 500 mg was evaluated. The dose regimen consisted of an initial rapid loading infusion, followed by a slower 6-hour loading infusion, and then an 18-hour maintenance infusion. The maintenance infusion was continued up to hour 48. Additional 10-minute infusions of 150 mg intravenous amiodarone were given for "breakthrough" VT/VF more frequently to the 125 mg dose group, thereby considerably reducing the planned 8-fold differences in total dose to 1.8- and 2.6-fold, respectively, in the two studies.
The prospectively defined primary efficacy end point was the rate of VT/VF episodes per hour. For both studies, the median rate was 0.02 episodes per hour in patients receiving the high dose and 0.07 episodes per hour in patients receiving the low dose, or approximately 0.5 versus 1.7 episodes per day (p=0.07, 2-sided, in both studies). In one study, the time to first episode of VT/VF was significantly prolonged (approximately 10 hours in patients receiving the low dose and 14 hours in patients receiving the high dose). In both studies, significantly fewer supplemental infusions were given to patients in the high-dose group. At the end of double-blind therapy or after 48 hours, all patients were given open access to whatever treatment (including intravenous amiodarone) was deemed necessary. Mortality was not affected in these studies. -
16 HOW SUPPLIED/STORAGE AND HANDLING
Amiodarone Hydrochloride Injection, USP is a sterile clear, pale yellow solution visually free from particulates available in glass vials packaged as follows:
150 mg per 3 mL (50 mg/mL):
3 mL in 3 mL Single Dose Vials,
packaged in cartons of 10 NDC: 55150-180-03
450 mg per 9 mL (50 mg/mL):
9 mL in 10 mL Single Dose Vials,
packaged in cartons of 10 NDC: 55150-181-09
900 mg per 18 mL (50 mg/mL):
18 mL in 20 mL Multiple Dose Vials,
packaged in cartons of 1 NDC: 55150-182-18
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light and excessive heat.
Use carton to protect contents from light until used.
The vial stopper is not made with natural rubber latex. -
17 PATIENT COUNSELING INFORMATION
Amiodarone has the potential to cause serious side effects that limit its use to life-threatening and hemodynamically unstable cardiac arrhythmias. Advise female patients to discontinue nursing while being treated with amiodarone, as breast-feeding could expose the nursing infant to a significant dose of the drug. Recommend that patients avoid grapefruit juice, over-the-counter cough medicines (which commonly contain dextromethorphan), and St. John's Wort. Inform patients that most manufacturers of corneal refractive laser surgery devices contraindicate corneal refractive laser surgery in patients taking amiodarone. Discuss the symptoms of hypo- and hyper-thyroidism, particularly if patients will be transitioned to oral amiodarone.
Distributed by:
AuroMedics Pharma LLC
279 Princeton-Hightstown Rd.
E. Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad - 500038
India
Revised: April 2017 -
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 150 mg per 3 mL (50 mg / mL) Container Label
Rx only NDC: 55150-180-03
Amiodarone
Hydrochloride Injection, USP
150 mg per 3 mL
(50 mg / mL)
For I.V. Use Only
MUST BE DILUTED
Sterile 3 mL Single Dose Vial
AUROMEDICS

-
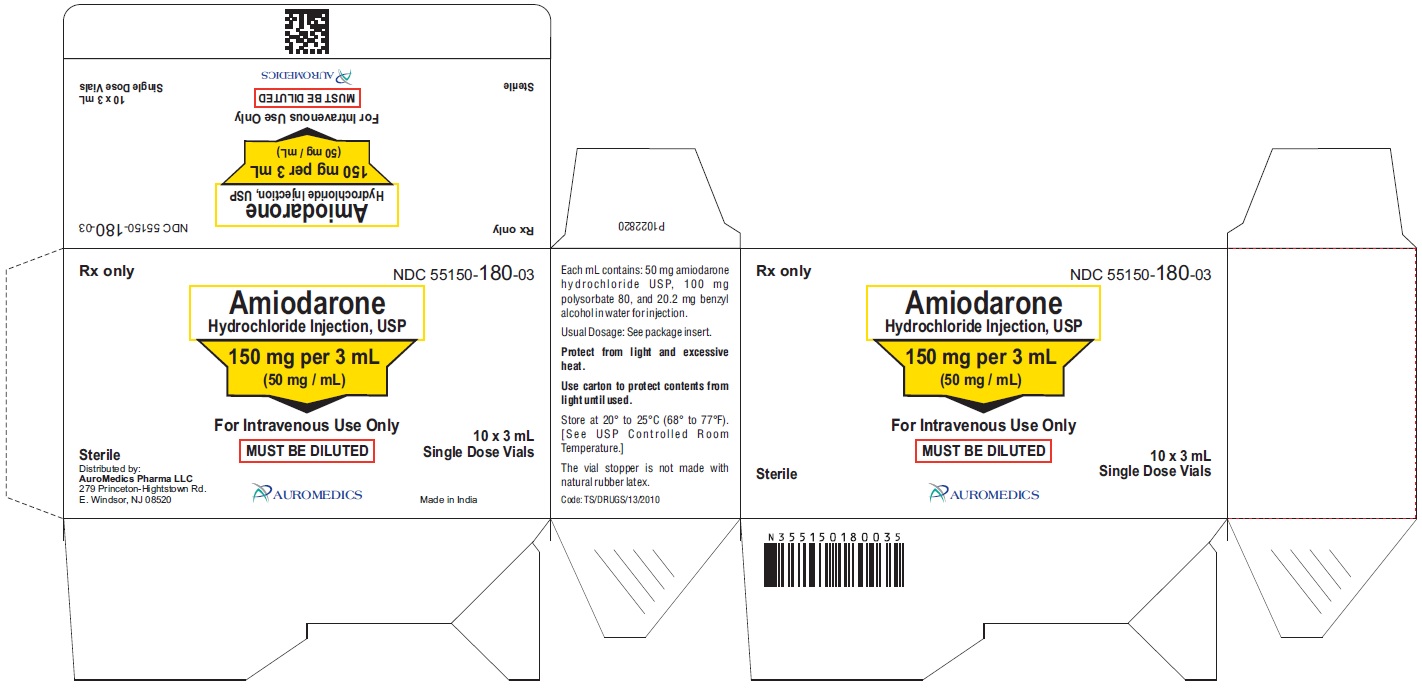
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 150 mg per 3 mL (50 mg / mL) Container-Carton (10 Vials)
Rx only NDC: 55150-180-03
Amiodarone
Hydrochloride Injection, USP
150 mg per 3 mL
(50 mg / mL)
For Intravenous Use Only
MUST BE DILUTED
Sterile 10 x 3 mL Single Dose Vials
AUROMEDICS
-
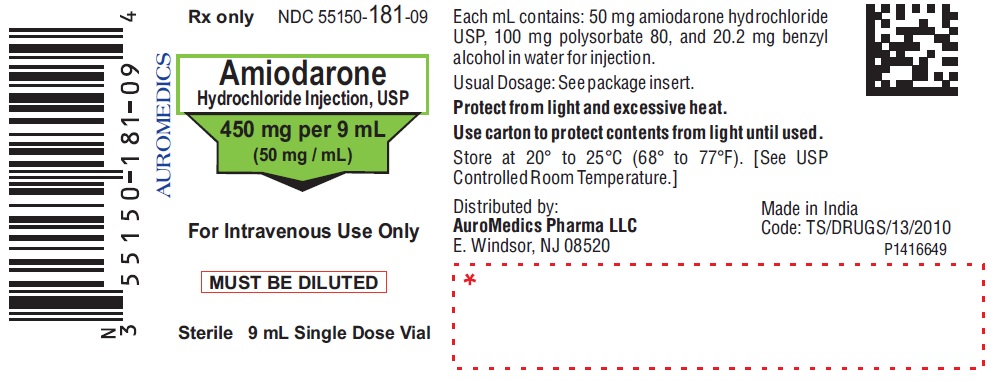
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 450 mg per 9 mL (50 mg / mL) Container Label
Rx only NDC: 55150-181-09
Amiodarone
Hydrochloride Injection, USP
450 mg per 9 mL
(50 mg / mL)
For Intravenous Use Only
MUST BE DILUTED
Sterile 9 mL Single Dose Vial
AUROMEDICS
-
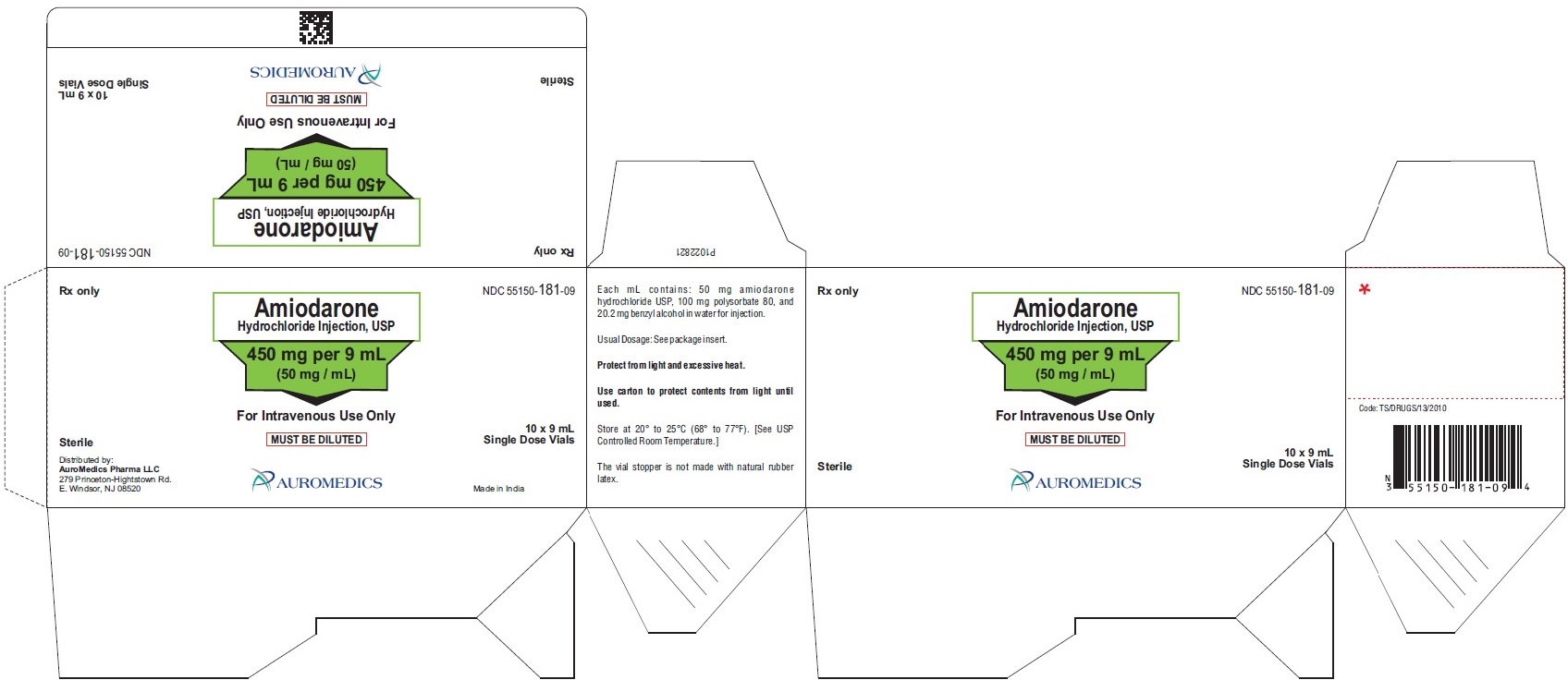
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 450 mg per 9 mL (50 mg / mL) Container-Carton (10 Vials)
Rx only NDC: 55150-181-09
Amiodarone
Hydrochloride Injection, USP
450 mg per 9 mL
(50 mg / mL)
For Intravenous Use Only
MUST BE DILUTED
Sterile 10 x 9 mL Single Dose Vials
AUROMEDICS
-
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 900 mg per 18 mL (50 mg / mL) Container Label
Rx only NDC: 55150-182-18
Amiodarone
Hydrochloride Injection, USP
900 mg per 18 mL
(50 mg / mL)
For Intravenous Use Only
MUST BE DILUTED
Sterile 18 mL Multiple Dose Vial
AUROMEDICS

-
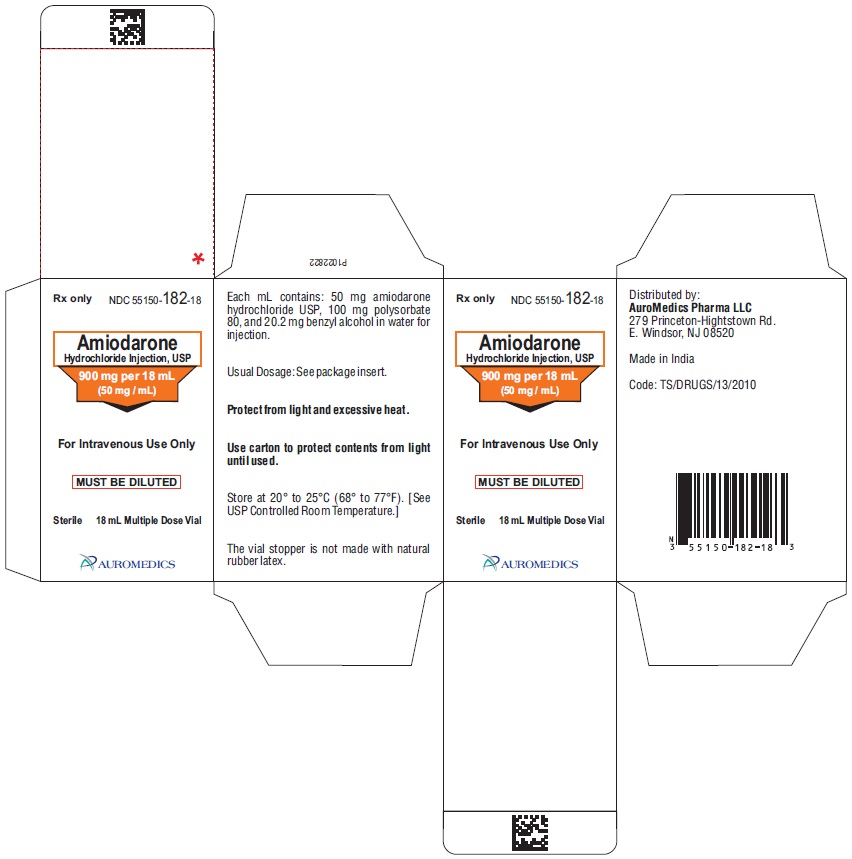
PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 900 mg per 18 mL (50 mg / mL) Container-Carton (1 Vial)
Rx only NDC: 55150-182-18
Amiodarone
Hydrochloride Injection, USP
900 mg per 18 mL
(50 mg / mL)
For Intravenous Use Only
MUST BE DILUTED
Sterile 18 mL Multiple Dose Vial
AUROMEDICS
-
INGREDIENTS AND APPEARANCE
AMIODARONE HYDROCHLORIDE
amiodarone hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55150-180 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMIODARONE HYDROCHLORIDE (UNII: 976728SY6Z) (AMIODARONE - UNII:N3RQ532IUT) AMIODARONE HYDROCHLORIDE 150 mg in 3 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) BENZYL ALCOHOL (UNII: LKG8494WBH) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55150-180-03 10 in 1 CARTON 10/25/2017 1 3 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204550 10/25/2017 AMIODARONE HYDROCHLORIDE
amiodarone hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55150-181 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMIODARONE HYDROCHLORIDE (UNII: 976728SY6Z) (AMIODARONE - UNII:N3RQ532IUT) AMIODARONE HYDROCHLORIDE 450 mg in 9 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) BENZYL ALCOHOL (UNII: LKG8494WBH) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55150-181-09 10 in 1 CARTON 10/25/2017 1 9 mL in 1 VIAL, SINGLE-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204550 10/25/2017 AMIODARONE HYDROCHLORIDE
amiodarone hydrochloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55150-182 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AMIODARONE HYDROCHLORIDE (UNII: 976728SY6Z) (AMIODARONE - UNII:N3RQ532IUT) AMIODARONE HYDROCHLORIDE 900 mg in 18 mL Inactive Ingredients Ingredient Name Strength POLYSORBATE 80 (UNII: 6OZP39ZG8H) BENZYL ALCOHOL (UNII: LKG8494WBH) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55150-182-18 1 in 1 CARTON 10/25/2017 1 18 mL in 1 VIAL, MULTI-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA204550 10/25/2017 Labeler - AuroMedics Pharma LLC (968961354) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650498244 ANALYSIS(55150-180, 55150-181, 55150-182) , MANUFACTURE(55150-180, 55150-181, 55150-182)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.