FEXOFENADINE HYDROCHLORIDE- fexofenadine tablet, film coated
Fexofenadine Hydrochloride by
Drug Labeling and Warnings
Fexofenadine Hydrochloride by is a Otc medication manufactured, distributed, or labeled by Mylan Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
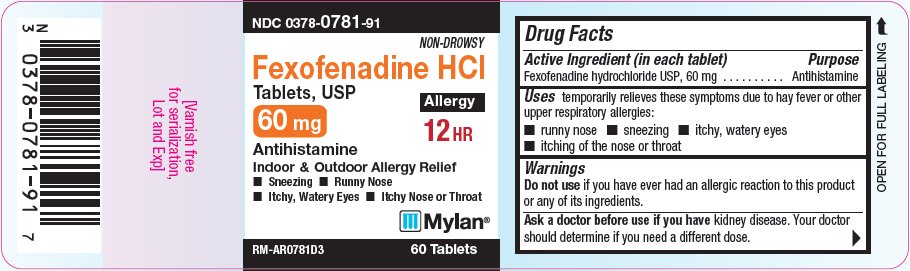
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
kidney disease. Your doctor should determine if you need a different dose.
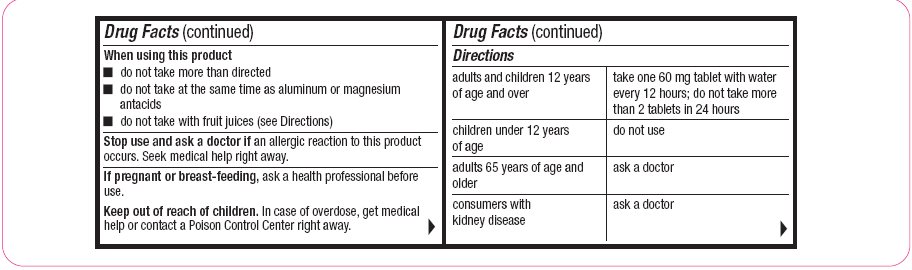
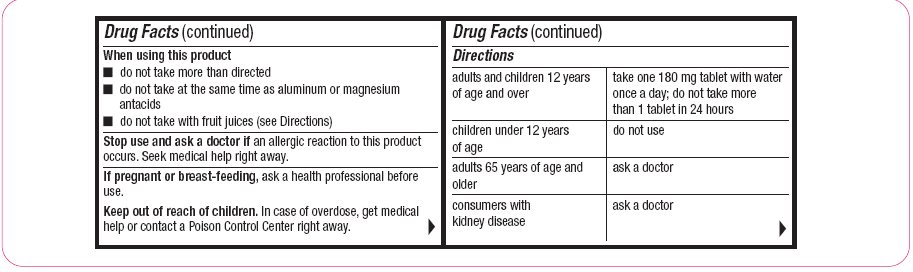
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
- Directions
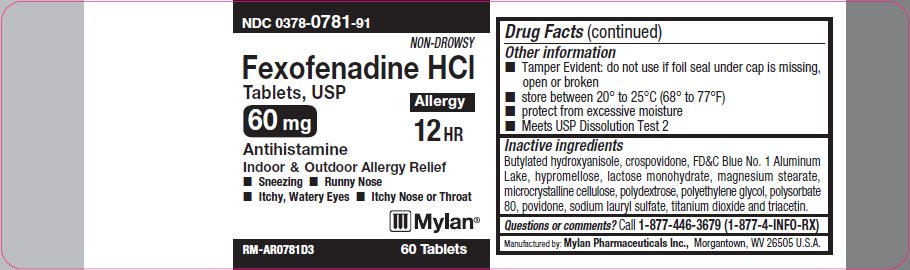
- Other information
- Inactive ingredients
- Questions or comments?
-
PRINCIPAL DISPLAY PANEL - 60 mg
NDC: 0378-0781-91
Non-Drowsy
Fexofenadine HCl
Tablets, USP60 mg
Allergy
12 HR
Antihistamine
Indoor/Outdoor Allergy Relief- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Nose or Throat
RM-AR0781D3 60 Tablets
Directions adults and children 12 years of age and over
take one 60 mg tablet with water every 12 hours; do not take more than 2 tablets in 24 hours
children under 12 years of age
do not use
adults 65 years of age and older
ask a doctor
consumers with kidney disease
ask a doctor
-
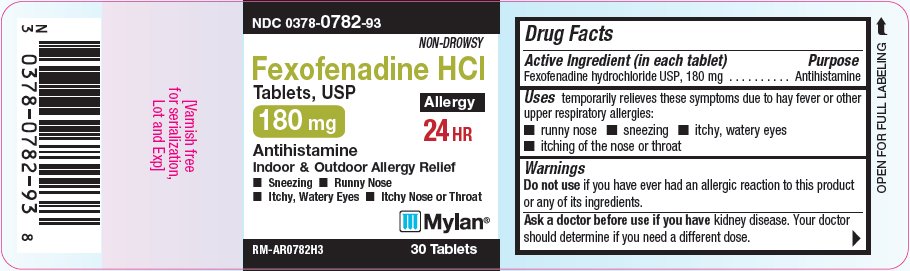
PRINCIPAL DISPLAY PANEL - 180 mg
NDC: 0378-0782-93
Non-Drowsy
Fexofenadine HCl
Tablets, USP180 mg
Allergy
24 HR
Antihistamine
Indoor/Outdoor Allergy Relief- Sneezing
- Runny Nose
- Itchy, Watery Eyes
- Itchy Nose or Throat
RM-AR0782H3 30 Tablets
Directions adults and children 12 years of age and over
take one 180 mg tablet with water once a day; do not take more than 1 tablet in 24 hours
children under 12 years of age
do not use
adults 65 years of age and older
ask a doctor
consumers with kidney disease
ask a doctor
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HYDROCHLORIDE
fexofenadine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0378-0781 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 60 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color BLUE Score no score Shape ROUND Size 8mm Flavor Imprint Code M;753 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0378-0781-91 60 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/25/2011 2 NDC: 0378-0781-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/25/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077081 08/25/2011 FEXOFENADINE HYDROCHLORIDE
fexofenadine tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0378-0782 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength BUTYLATED HYDROXYANISOLE (UNII: REK4960K2U) CROSPOVIDONE, UNSPECIFIED (UNII: 2S7830E561) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) SODIUM LAURYL SULFATE (UNII: 368GB5141J) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color BLUE Score no score Shape OVAL (caplet-shaped) Size 18mm Flavor Imprint Code M;755 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0378-0782-93 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/25/2011 2 NDC: 0378-0782-05 500 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 08/25/2011 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077081 08/25/2011 Labeler - Mylan Pharmaceuticals Inc. (059295980)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.