FEXOFENADINE HYDROCHLORIDE tablet, film coated
Fexofenadine Hydrochloride by
Drug Labeling and Warnings
Fexofenadine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Aphena Pharma Solutions - Tennessee, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use fexofenadine hydrochloride tablets USP safely and effectively. See full prescribing information for fexofenadine hydrochloride tablets USP.
Fexofenadine hydrochloride tablets USP for oral use.
Initial U.S. Approval: 1996INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
Patient Population Fexofenadine Hydrochloride Tablets USP (2.1) - * starting dose in patients with decreased renal function should be the recommended dose indicated above but administered once daily
- † dose not for use in patients with decreased renal function
Adults and children ≥ 12 years 60 mg twice daily* or 180 mg once daily† Children 6 to 11 years 30 mg twice daily* Children 2 to 5 years N/A Children 6 months to less than 2 years N/A - Fexofenadine hydrochloride tablets USP: take with water (2.1)
DOSAGE FORMS AND STRENGTHS
- Fexofenadine hydrochloride tablets USP: 30 mg, 60 mg, and 180 mg (3)
CONTRAINDICATIONS
Patients with known hypersensitivity to fexofenadine and any of the ingredients of fexofenadine hydrochloride tablets. (4)
ADVERSE REACTIONS
The most common adverse reactions (≥ 2%) in subjects age 12 years and older were headache, back pain, dizziness, stomach discomfort, and pain in extremity. In subjects aged 6 to 11 years, cough, upper respiratory tract infection, pyrexia and otitis media were more frequently reported. In subjects aged 6 months to 5 years, vomiting, diarrhea, somnolence/fatigue and rhinorrhea were more frequently reported. (6.1) Other adverse reactions have been reported. (6)
To report SUSPECTED ADVERSE REACTIONS, contact TEVA USA, PHARMACOVIGILANCE at tel: 1-888-838-2872, X6351 and drug.safety@tevausa.com or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
DRUG INTERACTIONS
- Antacids: Do not take at the same time as aluminum and magnesium containing antacids (7.1)
- Fruit juice: Take with water; not fruit juice
USE IN SPECIFIC POPULATIONS
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 7/2010
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Seasonal Allergic Rhinitis
1.2 Chronic Idiopathic Urticaria
2 DOSAGE AND ADMINISTRATION
2.1 Fexofenadine Hydrochloride Tablets USP
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Antacids
7.2 Erythromycin and Ketoconazole
7.3 Fruit Juices
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.3 Nursing Mothers
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Renal Impairment
8.7 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
14.1 Seasonal Allergic Rhinitis
14.2 Chronic Idiopathic Urticaria
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Fexofenadine Hydrochloride Tablets USP
Seasonal Allergic Rhinitis and Chronic Idiopathic Urticaria
Adults and Children 12 Years and Older: The recommended dose of fexofenadine hydrochloride tablets USP are 60 mg twice daily or 180 mg once daily with water. A dose of 60 mg once daily is recommended as the starting dose in patients with decreased renal function [see Clinical Pharmacology (12.3)].
Children 6 to 11 Years: The recommended dose of fexofenadine hydrochloride tablets USP are 30 mg twice daily with water. A dose of 30 mg once daily is recommended as the starting dose in pediatric patients with decreased renal function [see Clinical Pharmacology (12.3)].
-
3 DOSAGE FORMS AND STRENGTHS
Fexofenadine hydrochloride tablets USP are available in 30 mg, 60 mg, and 180 mg strengths. Fexofenadine hydrochloride tablets USP are coated with a peach colored film coating. Tablets have the following unique shape and identifiers: 30 mg tablets are capsule-shaped and have “93” on one side and “7251” on the other; 60 mg tablets are round and have “93” on one side and “7252” on the other; and 180 mg tablets are round and have “93” on one side and “7253” on the other.
-
4 CONTRAINDICATIONS
Fexofenadine hydrochloride tablets are contraindicated in patients with known hypersensitivity to fexofenadine and any of the ingredients of fexofenadine hydrochloride tablets. Rare cases of hypersensitivity reactions with manifestations such as angioedema, chest tightness, dyspnea, flushing and systemic anaphylaxis have been reported.
-
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety data described below reflect exposure to fexofenadine hydrochloride in 5083 patients in trials for allergic rhinitis and chronic idiopathic urticaria. In these trials, 3010 patients 12 years of age and older with seasonal allergic rhinitis were exposed to fexofenadine hydrochloride at doses of 20 to 240 mg twice daily or 120 to 180 mg once daily. A total of 646 patients 6 to 11 years of age with seasonal allergic rhinitis were exposed to fexofenadine hydrochloride at doses of 15 to 60 mg twice daily. The duration of treatment in these trials was 2 weeks. A total of 534 patients 6 months to 5 years of age with allergic rhinitis were exposed to fexofenadine hydrochloride at doses of 15 to 30 mg twice daily. The duration of treatment in these trials ranged from 1 day to 2 weeks. There were 893 patients 12 years of age and older with chronic idiopathic urticaria exposed to fexofenadine hydrochloride at doses of 20 to 240 mg twice daily or 180 mg once daily. The duration of treatment in these trials was 4 weeks.
Seasonal Allergic Rhinitis
Adults and Adolescents: In placebo-controlled seasonal allergic rhinitis clinical trials in subjects 12 years of age and older, 2439 subjects received fexofenadine hydrochloride capsules at doses of 20 mg to 240 mg twice daily. All adverse reactions that were reported by greater than 1% of subjects who received the recommended daily dose of fexofenadine hydrochloride (60 mg capsules twice daily) are listed in Table 1.
In another placebo-controlled clinical study in the United States, 571 subjects aged 12 years and older received fexofenadine hydrochloride tablets at doses of 120 or 180 mg once daily. Table 1 also lists adverse reactions that were reported by greater than 2% of subjects treated with fexofenadine hydrochloride tablets at doses of 180 mg once daily.
The incidence of adverse reactions, including somnolence/fatigue, was not dose-related and was similar across subgroups defined by age, gender, and race.
Table 1: Adverse Reactions in Subjects Aged 12 Years and Older Reported in Placebo-Controlled Seasonal Allergic Rhinitis Clinical Trials in the United States Twice-daily dosing with fexofenadine capsules at rates of greater than 1% Adverse reaction Fexofenadine 60 mg Twice Daily (n = 680) Frequency Placebo Twice Daily (n = 674) Frequency Dysmenorrhea 1.5% 0.3% Once-daily dosing with fexofenadine hydrochloride tablets at rates of greater than 2% Adverse reaction Fexofenadine 180 mg Once Daily (n = 283) Frequency Placebo(n = 293) Frequency Headache 10.3% 7.2% Back Pain 2.5% 1.4% The frequency and magnitude of laboratory abnormalities were similar in fexofenadine hydrochloride- and placebo-treated subjects.
Pediatrics:Table 2 lists adverse reactions in subjects aged 6 years to 11 years of age which were reported by greater than 2% of subjects treated with fexofenadine hydrochloride tablets at a dose of 30 mg twice daily in placebo-controlled seasonal allergic rhinitis studies in the United States and Canada.
Table 2: Adverse Reactions Reported in Placebo-Controlled Seasonal Allergic Rhinitis Studies in Pediatric Subjects Aged 6 Years to 11 Years in the United States and Canada at Rates of Greater Than 2% Adverse reaction Fexofenadine 30 mg Twice Daily(n = 209)Frequency Placebo(n = 229)Frequency Cough 3.8% 1.3% Upper Respiratory Tract Infection 2.9% 0.9% Pyrexia 2.4% 0.9% Otitis Media 2.4% 0.0% Table 3 lists adverse reactions in subjects 6 months to 5 years of age which were reported by greater than 2% of subjects treated with fexofenadine hydrochloride in 3 open single- and multiple-dose pharmacokinetic studies and 3 placebo-controlled safety studies with fexofenadine hydrochloride capsule content (484 subjects) and suspension (50 subjects) at doses of 15 mg (108 subjects) and 30 mg (426 subjects) given twice a day.
Table 3: Adverse Reactions Reported in Placebo-Controlled Studies in Pediatric Subjects With Allergic Rhinitis Aged 6 Months to 5 Years of Age at Rates Greater Than 2% Adverse reaction Fexofenadine 15 mg Twice Daily(n = 108)Frequency Fexofenadine 30 mg Twice Daily(n = 426)Frequency FexofenadineTotal Twice Daily(n = 534)Frequency Placebo(n = 430)Frequency Vomiting 12.0% 4.2% 5.8% 8.6% Diarrhea 3.7% 2.8% 3.0% 2.6% Somnolence/Fatigue 2.8% 0.9% 1.3% 0.2% Rhinorrhea 0.9% 2.1% 1.9% 0.9% Chronic Idiopathic Urticaria
Adverse reactions reported by subjects 12 years of age and older in placebo-controlled chronic idiopathic urticaria studies were similar to those reported in placebo-controlled seasonal allergic rhinitis studies.
In placebo-controlled chronic idiopathic urticaria clinical trials, 726 subjects 12 years of age and older received fexofenadine hydrochloride tablets at doses of 20 to 240 mg twice daily. Table 4 lists adverse reactions in subjects aged 12 years and older which were reported by greater than 2% of subjects treated with fexofenadine hydrochloride 60 mg tablets twice daily in controlled clinical studies in the United States and Canada.
In a placebo-controlled clinical study in the United States, 167 subjects aged 12 years and older received fexofenadine hydrochloride 180 mg tablets. Table 4 also lists adverse reactions that were reported by greater than 2% of subjects treated with fexofenadine hydrochloride tablets at doses of 180 mg once daily.
Table 4: Adverse Reactions Reported in Subjects 12 Years of Age and Older in Placebo-Controlled Chronic Idiopathic Urticaria Studies Twice-daily dosing with fexofenadine hydrochloride in studies in the United States and Canada at rates of greater than 2% Adverse reaction Fexofenadine 60 mg Twice Daily (n = 191) Frequency Placebo (n = 183) Frequency Dizziness 2.1% 1.1% Back Pain 2.1% 1.1% Stomach discomfort 2.1% 0.6% Pain in extremity 2.1% 0.0% Once-daily dosing with fexofenadine hydrochloride in a study in the United States at rates of greater than 2% Adverse reaction Fexofenadine 180 mg Once Daily (n = 167) Frequency Placebo (n = 92) Frequency Headache 4.8% 3.3% The safety of fexofenadine hydrochloride in the treatment of chronic idiopathic urticaria in pediatric patients 6 months to 11 years of age is based on the safety profile of fexofenadine hydrochloride in adults and pediatric patients at doses equal to or higher than the recommended dose [see Use in Specific Populations (8.4)].
6.2 Postmarketing Experience
In addition to the adverse reactions reported during clinical studies and listed above, the following adverse events have been identified during post-approval use of fexofenadine hydrochloride. Because these events are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure. Events that have been reported rarely during postmarketing experience include: insomnia, nervousness, sleep disorders or paroniria, and hypersensitivity reactions (including anaphylaxis, urticaria, angioedema, chest tightness, dyspnea, flushing, pruritus, and rash).
-
7 DRUG INTERACTIONS
7.1 Antacids
Fexofenadine hydrochloride should not be taken closely in time with aluminum and magnesium containing antacids. In healthy adult subjects, administration of 120 mg of fexofenadine hydrochloride (2 x 60 mg capsule) within 15 minutes of an aluminum and magnesium containing antacid (Maalox®) decreased fexofenadine AUC by 41% and Cmax by 43%.
7.2 Erythromycin and Ketoconazole
Fexofenadine has been shown to exhibit minimal (ca. 5%) metabolism. However, coadministration of fexofenadine hydrochloride with either ketoconazole or erythromycin led to increased plasma concentrations of fexofenadine in healthy adult subjects. Fexofenadine had no effect on the pharmacokinetics of either erythromycin or ketoconazole. In 2 separate studies in healthy adult subjects, fexofenadine hydrochloride 120 mg twice daily (240 mg total daily dose) was coadministered with either erythromycin 500 mg every 8 hours or ketoconazole 400 mg once daily under steady-state conditions to healthy adult subjects (n = 24, each study). No differences in adverse events or QTc interval were observed when subjects were administered fexofenadine hydrochloride alone or in combination with either erythromycin or ketoconazole. The findings of these studies are summarized in the following table:
Table 5: Effects on Steady-State Fexofenadine Pharmacokinetics After 7 Days of Coadministration With Fexofenadine Hydrochloride 120 mg Every 12 Hours in Healthy Adult Subjects (n = 24) Concomitant Drug CmaxSS(Peak plasma concentration) AUCss(0-12h)(Extent of systemic exposure) Erythromycin (500 mg every 8 hrs) +82% +109% Ketoconazole (400 mg once daily) +135% +164% The changes in plasma levels were within the range of plasma levels achieved in adequate and well-controlled clinical trials.
The mechanism of these interactions has been evaluated in in vitro, in situ, and in vivo animal models. These studies indicate that ketoconazole or erythromycin coadministration enhances fexofenadine gastrointestinal absorption. This observed increase in the bioavailability of fexofenadine may be due to transport-related effects, such as p-glycoprotein. In vivo animal studies also suggest that in addition to enhancing absorption, ketoconazole decreases fexofenadine gastrointestinal secretion, while erythromycin may also decrease biliary excretion.
7.3 Fruit Juices
Fruit juices such as grapefruit, orange and apple may reduce the bioavailability and exposure of fexofenadine. This is based on the results from 3 clinical studies using histamine induced skin wheals and flares coupled with population pharmacokinetic analysis. The size of wheal and flare were significantly larger when fexofenadine hydrochloride was administered with either grapefruit or orange juices compared to water. Based on the literature reports, the same effects may be extrapolated to other fruit juices such as apple juice. The clinical significance of these observations is unknown. In addition, based on the population pharmacokinetics analysis of the combined data from grapefruit and orange juices studies with the data from a bioequivalence study, the bioavailability of fexofenadine was reduced by 36%. Therefore, to maximize the effects of fexofenadine, it is recommended that fexofenadine hydrochloride tablets should be taken with water [see Clinical Pharmacology (12.3) and Dosage and Administration (2.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects
Pregnancy Category C. There was no evidence of teratogenicity in rats or rabbits at oral doses of terfenadine up to 300 mg/kg (which led to fexofenadine exposures that were approximately 4 and 30 times, respectively, the exposure at the maximum recommended human daily oral dose of 180 mg of fexofenadine hydrochloride based on comparison of AUCs).
In mice, no adverse effects and no teratogenic effects during gestation were observed with fexofenadine hydrochloride at oral doses up to 3730 mg/kg (which led to fexofenadine exposures that were approximately 15 times the exposure at the maximum recommended human daily oral dose of 180 mg of fexofenadine hydrochloride based on comparison of AUCs).
There are no adequate and well controlled studies in pregnant women. Fexofenadine hydrochloride should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Dose-related decreases in pup weight gain and survival were observed in rats exposed to an oral dose of 150 mg/kg of terfenadine (which led to fexofenadine exposures that were approximately 3 times the exposure at the maximum recommended human daily oral dose of 180 mg of fexofenadine hydrochloride based on comparison of AUCs).
8.3 Nursing Mothers
It is not known if fexofenadine is excreted in human milk. There are no adequate and well-controlled studies in women during lactation. Because many drugs are excreted in human milk, caution should be exercised when fexofenadine hydrochloride is administered to a nursing woman.
8.4 Pediatric Use
The recommended doses of fexofenadine hydrochloride in pediatric patients 6 months to 11 years of age are based on cross-study comparison of the pharmacokinetics of fexofenadine in adults and pediatric subjects and on the safety profile of fexofenadine hydrochloride in both adult and pediatric subjects at doses equal to or higher than the recommended doses. The safety and effectiveness of fexofenadine hydrochloride in pediatric patients under 6 months of age have not been established.
The safety of fexofenadine hydrochloride is based on the administration of fexofenadine hydrochloride tablets at a dose of 30 mg twice daily demonstrated in 438 pediatric subjects 6 years to 11 years of age in 2 placebo-controlled 2 week seasonal allergic rhinitis trials. The safety of fexofenadine hydrochloride at doses of 15 mg and 30 mg given once and twice a day has been demonstrated in 969 pediatric subjects (6 months to 5 years of age) with allergic rhinitis in 3 pharmacokinetic studies and 3 safety studies. The safety of fexofenadine hydrochloride for the treatment of chronic idiopathic urticaria in subjects 6 months to 11 years of age is based on cross-study comparison of the pharmacokinetics of fexofenadine hydrochloride in adult and pediatric subjects and on the safety profile of fexofenadine in both adult and pediatric subjects at doses equal to or higher than the recommended dose.
The effectiveness of fexofenadine hydrochloride for the treatment of seasonal allergic rhinitis in subjects 6 to 11 years of age was demonstrated in 1 trial (n = 411) in which fexofenadine hydrochloride tablets 30 mg twice daily significantly reduced total symptom scores compared to placebo, along with extrapolation of demonstrated efficacy in subjects aged 12 years and above, and the pharmacokinetic comparisons in adults and children. The effectiveness of fexofenadine hydrochloride 30 mg twice daily for the treatment of seasonal allergic rhinitis in patients 2 to 5 years of age is based on the pharmacokinetic comparisons in adult and pediatric subjects and an extrapolation of the demonstrated efficacy of fexofenadine hydrochloride in adult subjects with this condition and the likelihood that the disease course, pathophysiology, and the drug’s effect are substantially similar in pediatric patients to those in adult patients. The effectiveness of fexofenadine hydrochloride for the treatment of chronic idiopathic urticaria in patients 6 months to 11 years of age is based on the pharmacokinetic comparisons in adults and children and an extrapolation of the demonstrated efficacy of fexofenadine hydrochloride in adults with this condition and the likelihood that the disease course, pathophysiology and the drug’s effect are substantially similar in children to that of adult patients. Administration of a 15 mg dose of fexofenadine hydrochloride to pediatric subjects 6 months to less than 2 years of age and a 30 mg dose to pediatric subjects 2 to 11 years of age produced exposures comparable to those seen with a dose of 60 mg administered to adults.
8.5 Geriatric Use
Clinical studies of fexofenadine hydrochloride tablets and capsules did not include sufficient numbers of subjects aged 65 years and over to determine whether this population responds differently from younger subjects. Other reported clinical experience has not identified differences in responses between the geriatric and younger subjects. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
Based on increases in bioavailability and half-life, a dose of 60 mg once daily is recommended as the starting dose in adult patients with decreased renal function (mild, moderate or severe renal impairment). For pediatric patients with decreased renal function (mild, moderate or severe renal impairment), the recommended starting dose of fexofenadine is 30 mg once daily for patients 2 to 11 years of age and 15 mg once daily for patients 6 months to less than 2 years of age [see Clinical Pharmacology (12.3)].
-
10 OVERDOSAGE
Dizziness, drowsiness, and dry mouth have been reported with fexofenadine hydrochloride overdose. Single doses of fexofenadine hydrochloride up to 800 mg (6 healthy subjects at this dose level), and doses up to 690 mg twice daily for 1 month (3 healthy subjects at this dose level) or 240 mg once daily for 1 year (234 healthy subjects at this dose level) were administered without the development of clinically significant adverse events as compared to placebo.
In the event of overdose, consider standard measures to remove any unabsorbed drug. Symptomatic and supportive treatment is recommended. Following administration of terfenadine, hemodialysis did not effectively remove fexofenadine, the major active metabolite of terfenadine, from blood (up to 1.7% removed).
No deaths occurred at oral doses of fexofenadine hydrochloride up to 5000 mg/kg in mice (110 times the maximum recommended daily oral dose in adults and children based on mg/m2) and up to 5000 mg/kg in rats (230 times the maximum recommended daily oral dose in adults and 210 times the maximum recommended daily oral dose in children based on mg/m2). Additionally, no clinical signs of toxicity or gross pathological findings were observed. In dogs, no evidence of toxicity was observed at oral doses up to 2000 mg/kg (300 times the maximum recommended daily oral dose in adults and 280 times the maximum recommended daily oral dose in children based on mg/m2).
-
11 DESCRIPTION
Fexofenadine hydrochloride, the active ingredient of fexofenadine hydrochloride tablets, is a histamine H1-receptor antagonist with the chemical name (±)-4-[1-hydroxy-4-[4-(hydroxydiphenylmethyl)-1-piperidinyl]-butyl]-α, α-dimethyl benzeneacetic acid hydrochloride. It has the following chemical structure:
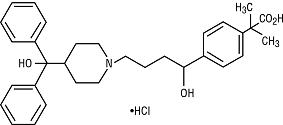
C32H39NO4HCl M.W. 538.13
Fexofenadine hydrochloride is a white to off-white crystalline powder. It is freely soluble in methanol and ethanol, slightly soluble in chloroform and water, and insoluble in hexane. Fexofenadine hydrochloride is a racemate and exists as a zwitterion in aqueous media at physiological pH.
Fexofenadine hydrochloride is formulated as a tablet for oral administration. Each tablet contains 30, 60, or 180 mg fexofenadine hydrochloride (depending on the dosage strength) and the following excipients: colloidal silicon dioxide, croscarmellose sodium, hypromellose, iron oxide black, iron oxide red, iron oxide yellow, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, and titanium dioxide.
Fexofenadine hydrochloride tablets meet USP Dissolution Test 3.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Fexofenadine hydrochloride, the major active metabolite of terfenadine, is an antihistamine with selective H1-receptor antagonist activity. Both enantiomers of fexofenadine hydrochloride displayed approximately equipotent antihistaminic effects. Fexofenadine hydrochloride inhibited antigen-induced bronchospasm in sensitized guinea pigs and histamine release from peritoneal mast cells in rats. The clinical significance of these findings is unknown. In laboratory animals, no anticholinergic or alpha1-adrenergic blocking effects were observed. Moreover, no sedative or other central nervous system effects were observed. Radiolabeled tissue distribution studies in rats indicated that fexofenadine does not cross the blood-brain barrier.
12.2 Pharmacodynamics
Wheal and Flare: Human histamine skin wheal and flare studies in adults following single and twice daily doses of 20 and 40 mg fexofenadine hydrochloride demonstrated that the drug exhibits an antihistamine effect by 1 hour, achieves maximum effect at 2 to 3 hours, and an effect is still seen at 12 hours. There was no evidence of tolerance to these effects after 28 days of dosing. The clinical significance of these observations is unknown.
Histamine skin wheal and flare studies in 7 to 12 year old subjects showed that following a single dose of 30 or 60 mg, antihistamine effect was observed at 1 hour and reached a maximum by 3 hours. Greater than 49% inhibition of wheal area, and 74% inhibition of flare area were maintained for 8 hours following the 30 and 60 mg dose.
No statistically significant increase in mean QTc interval compared to placebo was observed in 714 adult subjects with seasonal allergic rhinitis given fexofenadine hydrochloride capsules in doses of 60 to 240 mg twice daily for 2 weeks. Pediatric subjects from 2 placebo-controlled trials (n = 855) treated with up to 60 mg fexofenadine hydrochloride twice daily demonstrated no significant treatment- or dose-related increases in QTc. In addition, no statistically significant increase in mean QTc interval compared to placebo was observed in 40 healthy adult subjects given fexofenadine hydrochloride as an oral solution at doses up to 400 mg twice daily for 6 days, or in 230 healthy adult subjects given fexofenadine hydrochloride 240 mg once daily for 1 year. In subjects with chronic idiopathic urticaria, there were no clinically relevant differences for any ECG intervals, including QTc, between those treated with fexofenadine hydrochloride 180 mg once daily (n = 163) and those treated with placebo (n = 91) for 4 weeks.
12.3 Pharmacokinetics
The pharmacokinetics of fexofenadine hydrochloride in subjects with seasonal allergic rhinitis and subjects with chronic urticaria were similar to those in healthy subjects.
Absorption:
Fexofenadine hydrochloride was absorbed following oral administration of a single dose of two 60 mg capsules to healthy male subjects with a mean time to maximum plasma concentration occurring at 2.6 hours post-dose. After administration of a single 60 mg capsule to healthy adult subjects, the mean maximum plasma concentration (Cmax) was 131 ng/mL. Following single dose oral administrations of either the 60 and 180 mg tablet to healthy adult male subjects, mean Cmax were 142 and 494 ng/mL, respectively. The tablet formulations are bioequivalent to the capsule when administered at equal doses. Fexofenadine hydrochloride pharmacokinetics are linear for oral doses up to a total daily dose of 240 mg (120 mg twice daily). The administration of the 60 mg capsule contents mixed with applesauce did not have a significant effect on the pharmacokinetics of fexofenadine in adults. Coadministration of 180 mg fexofenadine hydrochloride tablet with a high fat meal decreased the mean area under the curve (AUC) and (Cmax) of fexofenadine by 21 and 20% respectively.
Distribution:
Fexofenadine hydrochloride is 60% to 70% bound to plasma proteins, primarily albumin and α1-acid glycoprotein.
Metabolism:
Approximately 5% of the total dose of fexofenadine hydrochloride was eliminated by hepatic metabolism.
Elimination:
The mean elimination half-life of fexofenadine was 14.4 hours following administration of 60 mg twice daily in healthy adult subjects.
Human mass balance studies documented a recovery of approximately 80% and 11% of the [14C] fexofenadine hydrochloride dose in the feces and urine, respectively. Because the absolute bioavailability of fexofenadine hydrochloride has not been established, it is unknown if the fecal component represents primarily unabsorbed drug or is the result of biliary excretion.
Special Populations:
Pharmacokinetics in renally and hepatically impaired subjects and geriatric subjects, obtained after a single dose of 80 mg fexofenadine hydrochloride, were compared to those from healthy subjects in a separate study of similar design.
Renally Impaired:
In subjects with mild to moderate (creatinine clearance 41 to 80 mL/min) and severe (creatinine clearance 11 to 40 mL/min) renal impairment, peak plasma concentrations of fexofenadine were 87% and 111% greater, respectively, and mean elimination half-lives were 59% and 72% longer, respectively, than observed in healthy subjects. Peak plasma concentrations in subjects on dialysis (creatinine clearance ≤ 10 mL/min) were 82% greater and half-life was 31% longer than observed in healthy subjects. Based on increases in bioavailability and half-life, a dose of 60 mg once daily is recommended as the starting dose in adult patients with decreased renal function. For pediatric patients with decreased renal function, the recommended starting dose of fexofenadine is 30 mg once daily for patients 2 to 11 years of age and 15 mg once daily for patients 6 months to less than 2 years of age.
Hepatically Impaired:
The pharmacokinetics of fexofenadine hydrochloride in subjects with hepatic impairment did not differ substantially from that observed in healthy subjects.
Geriatric Subjects:
In older subjects (≥ 65 years old), peak plasma levels of fexofenadine were 99% greater than those observed in younger subjects (< 65 years old). Mean fexofenadine elimination half-lives were similar to those observed in younger subjects.
Pediatric Subjects:
A population pharmacokinetic analysis was performed with data from 77 pediatric subjects (6 months to 12 years of age) with allergic rhinitis and 136 adult subjects. The individual apparent oral clearance estimates of fexofenadine were on average 44% and 36% lower in pediatric subjects 6 to 12 years (n = 14) and 2 to 5 years of age (n = 21), respectively, compared to adult subjects.
Administration of a 15 mg dose of fexofenadine hydrochloride to pediatric subjects 6 months to less than 2 years of age and a 30 mg dose to pediatric subjects 2 to 11 years of age produced exposures comparable to those seen with a dose of 60 mg administered to adults.
Effect of Gender:
Across several trials, no clinically significant gender-related differences were observed in the pharmacokinetics of fexofenadine hydrochloride.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of fexofenadine was assessed using terfenadine studies with adequate fexofenadine exposure (based on plasma area-under-the-concentration vs. time [AUC] values). No evidence of carcinogenicity was observed in an 18 month study in mice and in a 24 month study in rats at oral doses up to 150 mg/kg of terfenadine (which led to fexofenadine exposures that were approximately 3 and 5 times the exposure at the maximum recommended daily oral dose of fexofenadine hydrochloride in adults [180 mg] and children [60 mg] respectively).
In in vitro (Bacterial Reverse Mutation, CHO/HGPRT Forward Mutation, and Rat Lymphocyte Chromosomal Aberration assays) and in vivo (Mouse Bone Marrow Micronucleus assay) tests, fexofenadine hydrochloride revealed no evidence of mutagenicity.
In rat fertility studies, dose-related reductions in implants and increases in postimplantation losses were observed at an oral dose of 150 mg/kg of terfenadine (which led to fexofenadine exposures that were approximately 3 times the exposure at the maximum recommended human daily oral dose of 180 mg of fexofenadine hydrochloride based on comparison of AUCs). In mice, fexofenadine hydrochloride produced no effect on male or female fertility at average oral doses up to 4438 mg/kg (which led to fexofenadine exposures that were approximately 13 times the exposure at the maximum recommended human daily oral dose of 180 mg of fexofenadine hydrochloride based on comparison of AUCs).
13.2 Animal Toxicology and/or Pharmacology
In dogs (30 mg/kg/orally twice daily for 5 days) and rabbits (10 mg/kg, intravenously over 1 hour), fexofenadine hydrochloride did not prolong QTc. In dogs, the plasma fexofenadine concentration was approximately 9 times the therapeutic plasma concentrations in adults receiving the maximum recommended human daily oral dose of 180 mg. In rabbits, the plasma fexofenadine concentration was approximately 20 times the therapeutic plasma concentration in adults receiving the maximum recommended human daily oral dose of 180 mg. No effect was observed on calcium channel current, delayed K+ channel current, or action potential duration in guinea pig myocytes, or on the delayed rectifier K+ channel cloned from human heart at concentrations up to 1 x 10-5 M of fexofenadine.
-
14 CLINICAL STUDIES
14.1 Seasonal Allergic Rhinitis
Adults: In three 2 week, multicenter, randomized, double-blind, placebo-controlled trials in subjects 12 to 68 years of age with seasonal allergic rhinitis (n = 1634), fexofenadine hydrochloride 60 mg twice daily significantly reduced total symptom scores (the sum of the individual scores for sneezing, rhinorrhea, itchy nose/palate/throat, itchy/watery/red eyes) compared to placebo. Statistically significant reductions in symptom scores were observed following the first 60 mg dose, with the effect maintained throughout the 12 hour interval. In these studies, there was no additional reduction in total symptom scores with higher doses of fexofenadine hydrochloride up to 240 mg twice daily.
In one 2 week, multicenter, randomized, double-blind clinical trial in subjects 12 to 65 years of age with seasonal allergic rhinitis (n = 863), fexofenadine hydrochloride 180 mg once daily significantly reduced total symptom scores (the sum of the individual scores for sneezing, rhinorrhea, itchy nose/palate/throat, itchy/watery/red eyes) compared to placebo. Although the number of subjects in some of the subgroups was small, there were no significant differences in the effect of fexofenadine hydrochloride across subgroups of subjects defined by gender, age, and race. Onset of action for reduction in total symptom scores, excluding nasal congestion, was observed at 60 minutes compared to placebo following a single 60 mg fexofenadine hydrochloride dose administered to subjects with seasonal allergic rhinitis who were exposed to ragweed pollen in an environmental exposure unit. In 1 clinical trial conducted with fexofenadine hydrochloride 60 mg capsules, and in 1 clinical trial conducted with fexofenadine hydrochloride and pseudoephedrine hydrochloride extended-release tablets (12 hour formulation), onset of action was seen within 1 to 3 hours.
Pediatrics: Two 2 week, multicenter, randomized, placebo-controlled, double-blind trials in 877 pediatric subjects 6 to 11 years of age with seasonal allergic rhinitis were conducted at doses of 15, 30, and 60 mg (tablets) twice daily. In 1 of these 2 studies, conducted in 411 pediatric subjects, all 3 doses of fexofenadine hydrochloride significantly reduced total symptom scores (the sum of the individual scores for sneezing, rhinorrhea, itchy nose/palate/throat, itchy/watery/red eyes) compared to placebo, however, a dose-response relationship was not seen. The 60 mg twice daily dose did not provide any additional benefit over the 30 mg twice daily dose in pediatric subjects 6 to 11 years of age.
Administration of a 30 mg dose to pediatric subjects 2 to 11 years of age produced exposures comparable to those seen with a dose of 60 mg administered to adults [see Clinical Pharmacology (12.3)].
14.2 Chronic Idiopathic Urticaria
Two 4 week, multicenter, randomized, double-blind, placebo-controlled clinical trials compared four different doses of fexofenadine hydrochloride tablet (20, 60, 120, and 240 mg twice daily) to placebo in subjects aged 12 to 70 years with chronic idiopathic urticaria (n = 726). Efficacy was demonstrated by a significant reduction in mean pruritus scores (MPS), mean number of wheals (MNW), and mean total symptom scores (MTSS, the sum of the MPS and MNW score). Although all 4 doses were significantly superior to placebo, symptom reduction was greater and efficacy was maintained over the entire 4 week treatment period with fexofenadine hydrochloride doses of ≥ 60 mg twice daily. However, no additional benefit of the 120 or 240 mg fexofenadine hydrochloride twice daily dose was seen over the 60 mg twice daily dose in reducing symptom scores. There were no significant differences in the effect of fexofenadine hydrochloride across subgroups of subjects defined by gender, age, weight, and race.
In one 4 week, multicenter, randomized, double-blind, placebo-controlled clinical trial in subjects 12 years of age and older with chronic idiopathic urticaria (n = 259), fexofenadine hydrochloride 180 mg once daily significantly reduced the mean number of wheals (MNW), the mean pruritus score (MPS), and the mean total symptom score (MTSS, the sum of the MPS and MNW scores). Similar reductions were observed for mean number of wheals and mean pruritus score at the end of the 24 hour dosing interval. Symptom reduction was greater with fexofenadine hydrochloride 180 mg than with placebo. Improvement was demonstrated within 1 day of treatment with fexofenadine hydrochloride 180 mg and was maintained over the entire 4 week treatment period. There were no significant differences in the effect of fexofenadine hydrochloride across subgroups of subjects defined by gender, age, and race.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Repackaged by Aphena Pharma Solutions - TN.
See Repackaging Information for available configurations.
Fexofenadine hydrochloride tablets USP are available as follows:
30 mg – peach, capsule-shaped, film-coated tablets debossed with “93” on one side and “7251” on the other side, in bottles of 100.
60 mg – peach, round, film-coated tablets debossed with “93” on one side and “7252” on the other side, in bottles of 100 and 500.
180 mg – peach, round, film-coated tablets debossed with “93” on one side and “7253” on the other side, in bottles of 100 and 500.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature]. Protect from excessive moisture.
Dispense in a tight, light-resistant container as defined in the USP, with a child-resistant closure (as required).
-
17 PATIENT COUNSELING INFORMATION
Provide the following information to patients and parents/caregivers of pediatric patients taking fexofenadine hydrochloride tablets:
- Fexofenadine hydrochloride tablets are prescribed for the relief of symptoms of seasonal allergic rhinitis or for the relief of symptoms of chronic idiopathic urticaria (hives). Instruct patients to take fexofenadine hydrochloride tablets only as prescribed. Do not exceed the recommended dose. If any untoward effects occur while taking fexofenadine hydrochloride tablets, discontinue use and consult a doctor.
- Patients who are hypersensitive to any of the ingredients should not use these products.
- Patients who are pregnant or nursing should use these products only if the potential benefit justifies the potential risk to the fetus or nursing infant.
- Advise patients and parents/caregivers of pediatric patients to store the medication in a tightly closed container in a cool, dry place, away from small children.
- Advise patients and parents/caregivers not to take fexofenadine hydrochloride tablets with fruit juices.
- Advise patients to take the fexofenadine hydrochloride tablets with water.
Maalox® is a registered trademark of AVENTIS PHARMACEUTICALS PRODUCTS INC.
Manufactured In Israel By:
TEVA PHARMACEUTICAL IND. LTD.
Jerusalem, 91010, Israel
Manufactured For:
TEVA PHARMACEUTICALS USA
Sellersville, PA 18960
Rev. G 10/2009
-
Repackaging Information
Please reference the How Supplied section listed above for a description of individual tablets or capsules. This drug product has been received by Aphena Pharma - TN in a manufacturer or distributor packaged configuration and repackaged in full compliance with all applicable cGMP regulations. The package configurations available from Aphena are listed below:
Count 180mg 30 43353-244-30 Store between 20°-25°C (68°-77°F). See USP Controlled Room Temperature. Dispense in a tight light-resistant container as defined by USP. Keep this and all drugs out of the reach of children.
Repackaged by:

Cookeville, TN 38506
20120727KRW - PRINCIPAL DISPLAY PANEL - 180mg
-
INGREDIENTS AND APPEARANCE
FEXOFENADINE HYDROCHLORIDE
fexofenadine hydrochloride tablet, film coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 43353-244(NDC: 0093-7253) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FEXOFENADINE HYDROCHLORIDE (UNII: 2S068B75ZU) (FEXOFENADINE - UNII:E6582LOH6V) FEXOFENADINE HYDROCHLORIDE 180 mg Inactive Ingredients Ingredient Name Strength COLLOIDAL SILICON DIOXIDE (UNII: ETJ7Z6XBU4) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) HYPROMELLOSES (UNII: 3NXW29V3WO) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color ORANGE (peach) Score no score Shape ROUND Size 12mm Flavor Imprint Code 93;7253 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43353-244-30 30 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076447 07/01/2010 Labeler - Aphena Pharma Solutions - Tennessee, Inc. (128385585) Establishment Name Address ID/FEI Business Operations Aphena Pharma Solutions - Tennessee, Inc. 128385585 Repack(43353-244)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
