NAVELBINE- vinorelbine tartrate injection
NAVELBINE by
Drug Labeling and Warnings
NAVELBINE by is a Prescription medication manufactured, distributed, or labeled by Pierre Fabre Pharmaceuticals, Inc., Pierre Fabre Medicament, Pierre Fabre Medicament Production. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use NAVELBINE safely and effectively. See full prescribing information for NAVELBINE.
NAVELBINE® (vinorelbine) injection, for intravenous use
Initial U.S. Approval: 1994INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
Injection: 1 mL (10 mg/ 1 mL) and 5 mL (50 mg/ 5 mL) clear colorless to pale yellow solution in single-dose vial. (3)
CONTRAINDICATIONS
None (4)
WARNINGS AND PRECAUTIONS
- Hepatic toxicity: Monitor hepatic function prior to initiation and during treatment t ( 5.2)
- Severe constipation and bowel obstruction including necrosis and perforation, occur. Institute a prophylactic bowel regimen to mitigate potential constipation, bowel obstruction and/or paralytic ileus. ( 5.3)
- Extravasation can result in severe tissue injury, necrosis and/or thrombophlebitis. Immediately stop NAVELBINE and institute recommended management procedures ( 5.4)
- Neurologic toxicity: severe sensory and motor neuropathies occur. Monitor patients for new or worsening signs and symptoms of neuropathy. Discontinue for Grade 2 or greater neuropathy ( 5.5)
- Pulmonary toxicity and respiratory failure occur. Interrupt NAVELBINE in patients who develop unexplained dyspnea or have any evidence of pulmonary toxicity. Permanently discontinue for confirmed interstitial pneumonitis or ARDA ( 5.6)
- Embryo-fetal toxicity: Can cause fetal harm. Advise females of reproductive potential of potential risk to the fetus ( 5.7, 8.1, 8.3)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥ 20%) are leukopenia, neutropenia, anemia, increased aspartate aminotransferase, nausea, vomiting, constipation, asthenia, injection site reaction, and peripheral neuropathy ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Pierre Fabre Pharmaceuticals at 1- 855-PFPHARM (1-855-737-4276) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Inhibitors of CYP3A4: May cause earlier onset and/or increased severity of adverse reactions ( 7.1)
USE IN SPECIFIC POPULATIONS
- Lactation: Advise not to breastfeed ( 8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 1/2020
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: MYELOSUPPRESSION
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
2.2 Dose Modifications
2.3 Preparation and Administration
2.4 Procedures for Proper Handling and Disposal
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
5.2 Hepatic Toxicity
5.3 Severe Constipation and Bowel Obstruction
5.4 Extravasation and Tissue Injury
5.5 Neurologic Toxicity
5.6 Pulmonary Toxicity and Respiratory Failure
5.7 Embryo-Fetal Toxicity
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 CYP3A Inhibitors
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Combination Use with Cisplatin
14.2 Single Agent
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: MYELOSUPPRESSION
- Severe myelosuppression resulting in serious infection, septic shock, hospitalization and death can occur [see Warnings and Precautions (5.1)].
- Decrease the dose or withhold NAVELBINE in accord with recommended dose modifications [see Dosage and Administration (2.2)].
- 1 INDICATIONS AND USAGE
-
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage
In Combination with Cisplatin 100 mg/m2
- The recommended dose of NAVELBINE is 25 mg/m 2 administered as an intravenous injection or infusion over 6 to 10 minutes on Days 1, 8, 15, and 21 of a 28 day cycle in combination with cisplatin 100 mg/m 2 on Day 1 only of each 28 day cycle.
In Combination with Cisplatin 120 mg/m2
- The recommended dose of NAVELBINE is 30 mg/m 2 administered as an intravenous injection or infusion over 6 to 10 minutes once a week in combination with cisplatin 120 mg/m 2 on Days 1 and 29, then every 6 weeks.
Single-Agent
- The recommended dose of NAVELBINE is 30 mg/m 2 administered intravenously over 6 to 10 minutes once a week.
2.2 Dose Modifications
Myelosuppression
[see Warnings and Precautions (5.1)]
Hold or decrease the dose of NAVELBINE in patients with decreased neutrophil counts using the following schema.
Neutrophils on Day of Treatment (Cells/mm 3)
Percentage of Starting Dose of NAVELBINE
≥ 1,500
100%
1,000 to 1,499
50%
< 1,000
Do not administer NAVELBINE.
Repeat neutrophil count in one week.
If three consecutive weekly doses are held because
Neutrophil count is < 1,000 cells/mm3, discontinue NAVELBINE Note : For patients who experience fever and/or sepsis while neutrophil count is < 1,500 or had 2 consecutive weekly doses held due to neutropenia, subsequent doses of NAVELBINE should be:
> 1,500
75%
1,000 to 1,499
37.5%
< 1,000
Do not administer NAVELBINE. Repeat neutrophil count in one week.
Hepatic Impairment/Toxicity
[seeWarnings and Precautions (5.2) and Use in Specific Populations(8.6)]
Reduce NAVELBINE dose in patients with elevated serum total bilirubin concentration according to the following schema:
Serum total bilirubin concentration (mg/dl)
Percentage of Starting Dose of NAVELBINE
< 2.0
100%
2.1 to 3.0
50%
> 3.0
25%
Concurrent Myelosuppression and Hepatic Impairment/Toxicity
In patients with both myelosuppression and hepatic impairment/toxicity, administer the lower of the doses based on the corresponding starting dose of NAVELBINE determined from the above schemas.
Neurologic Toxicity
[see Warnings and Precautions (5.5)]
Discontinue NAVELBINE for for Common Terminology Criteria for Adverse Events (CTCAE) Grade 2 or higher peripheral neuropathy or autonomic neuropathy causing constipation.
2.3 Preparation and Administration
Preparation
Dilute NAVELBINE in an intravenous bag to a concentration between 0.5 mg/mL and 2 mg/mL. Use one of the following recommended solutions for dilution:
- 5% Dextrose Injection, USP
- 0.9% Sodium Chloride Injection, USP
- 0.45% Sodium Chloride Injection, USP
- 5% Dextrose and 0.45% Sodium Chloride Injection, USP
- Ringer's Injection, USP
- Lactated Ringer's Injection, USP
Stability and Storage Conditions of Diluted Solutions
Diluted NAVELBINE may be used for up to 24 hours under normal room light when stored in polyvinyl chloride bags at 5° to 30°C (41° to 86°F).
Administration
Administer diluted NAVELBINE over 6 to 10 minutes into the side port of a free-flowing intravenous line followed by flushing with at least 75 to 125 mL of one of the solutions.
NAVELBINE must only be administered intravenously. It is extremely important that the intravenous needle or catheter be properly positioned before any NAVELBINE is injected.
Parenteral drug products should be visually inspected for particulate matter and discoloration prior to administration whenever solution and container permit. If particulate matter is seen, NAVELBINE should not be administered.
Management of Suspected Extravasation
- If NAVELBINE leakage into surrounding tissue occurs or is suspected, immediately stop administration of NAVELBINE and initiate appropriate management measures in accordance with institutional policies [see Warnings and Precautions (5.4)].
2.4 Procedures for Proper Handling and Disposal
NAVELBINE is a cytotoxic drug. Follow applicable special handling and disposal procedures 1.
Exercise caution in handling and preparing the solution of NAVELBINE. The use of gloves is recommended. If the solution of NAVELBINE contacts the skin or mucosa, immediately wash the skin or mucosa thoroughly with soap and water.
Avoid contamination of the eye with NAVELBINE. If exposure occurs, flush the eyes with water immediately and thoroughly.
Discard unused portion.
- 3 DOSAGE FORMS AND STRENGTHS
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Myelosuppression
Myelosuppression manifested by neutropenia, anemia and thrombocytopenia occur with NAVELBINE as a single agent and in combination with cisplatin [see Adverse Reactions (6.1 and 6.2)]. Neutropenia is the major dose-limiting toxicity with NAVELBINE. Grade 3-4 neutropenia occurred in 53% of patients treated with NAVELBINE at 30 mg/m 2 per week. Dose adjustment due to myelosuppression occurred in 51% of patients (Study 2). In clinical trials with NAVELBINE administered at 30 mg/m 2 per week, neutropenia resulted in hospitalizations for pyrexia and/or sepsis in 8% of patients. Death due to sepsis occurred in 1% of patients. Neutropenia nadirs occur between 7 and 10 days after dosing with neutropenia count recovery usually occurring within the following 7 to 14 days.
Monitor complete blood counts prior to each dose of NAVELBINE. Do not administer NAVELBINE to patients with neutrophil counts <1,000 cells/mm 3. Adjustments in the dosage of NAVELBINE should be based on neutrophil counts obtained on the day of treatment [ see Dosage and Administration (2.2)].
5.2 Hepatic Toxicity
Drug-induced liver injury manifest by elevations of aspartate aminotransferase and bilirubin can occur in patients receiving NAVELBINE as a single agent or in combination with cytotoxic agents. Assess hepatic function prior to initiation of NAVELBINE and periodically during treatment. Reduce the dose of NAVELBINE for patients who develop elevations in total bilirubin > 2 times upper limit of normal [see Dosage and Administration (2.2)and Use in Specific Populations (8.6))].
5.3 Severe Constipation and Bowel Obstruction
Severe and fatal paralytic ileus, constipation, intestinal obstruction, necrosis, and perforation occur with NAVELBINE administration. Institute a prophylactic bowel regimen to mitigate potential constipation, bowel obstruction and/or paralytic ileus, considering adequate dietary fiber intake, hydration, and routine use of stool softeners.
5.4 Extravasation and Tissue Injury
Extravasation of NAVELBINE can result in severe irritation, local tissue necrosis and/or thrombophlebitis. If signs or symptoms of extravasation occur, immediately stop administration of NAVELBINE and institute recommended management procedures [see Dosage and Administration (2.2)and Adverse Reaction (6.1)].
5.5 Neurologic Toxicity
Sensory and motor neuropathies, including severe neuropathies, occur in patients receiving NAVELBINE. Monitor patients for new or worsening signs and symptoms of neuropathy such as paresthesia, hyperesthesia, hyporeflexia and muscle weakness while receiving NAVELBINE. Discontinue NAVELBINE for CTCAE Grade 2 or greater neuropathy [see Dosage and Administration (2.2) and Adverse Reaction (6.1)].
5.6 Pulmonary Toxicity and Respiratory Failure
Pulmonary toxicity, including severe acute bronchospasm, interstitial pneumonitis, acute respiratory distress syndrome (ARDS) occurs with use of NAVELBINE. Interstitial pneumonitis and ARDS included fatalities. The mean time to onset of interstitial pneumonitis and ARDS after vinorelbine administration was one week (range 3 to 8 days) [see Adverse Reactions (6.1)].
Interrupt NAVELBINE in patients who develop unexplained dyspnea, or have any evidence of pulmonary toxicity. Permanently discontinue NAVELBINE for confirmed interstitial pneumonitis or ARDS.
5.7 Embryo-Fetal Toxicity
Based on findings from animal studies and its mechanism of action, NAVELBINE can cause fetal harm when administered to a pregnant woman. In animal reproduction studies in mice and rabbits, embryo and fetal toxicity were observed with administration of vinorelbine at doses approximately 0.33 and 0.18 times the human therapeutic dose, respectively.
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with NAVELBINE and for 6 months after the final dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with NAVELBINE and for 3 months after the final dose [see Use in Specific Populations (8.1, 8.3)].
-
6 ADVERSE REACTIONS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Myelosuppression [see Warnings and Precautions (5.1)]
- Hepatic Toxicity [see Warning and Precautions (5.2)]
- Severe Constipation and Bowel Obstruction [see Warnings and Precautions (5.3)]
- Extravasation Tissue Injury [see Warnings and Precautions (5.4)]
- Neurologic Toxicity [see Warnings and Precautions (5.5)]
- Pulmonary Toxicity and Respiratory Failure [see Warnings and Precautions (5.6)) ]
6.1 Clinical Trials Experience
Because clinical trials are conducted under varying designs and in different patient populations, the adverse reaction rates reported in one clinical trial may not be easily compared to those rates reported in another clinical trial, and may not reflect the rates actually observed in clinical practice.
Single Agent
The data below reflect exposure to NAVELBINE as a single agent administered at a dose of 30 mg/m2 on a weekly basis to 365 patients enrolled in 3 controlled studies for metastatic NSCLC and advanced breast cancer. The population included 143 previously untreated metastatic NSCLC patients (Study 3) who received a median of 8 doses of NAVELBINE. The patients were aged 32 to 79 (median 61 years), 71% were male, 91% White, 48% had adenocarcinoma histology. The data also reflect exposure to NAVELBINE in 222 patients with previously treated advanced breast cancer who received a median of 10 doses of NAVELBINE. NAVELBINE is not indicated for the treatment of breast cancer.
Selected adverse reactions reported in these studies are provided in Tables 1 and 2. The most common adverse reactions (≥ 20%) of single agent NAVELBINE were leukopenia, neutropenia, anemia, Aspartate aminotransferase (AST) elevation, nausea, vomiting, constipation, asthenia, injection site reaction, and peripheral neuropathy. The most common (≥ 5%) Grade 3 or 4 adverse reactions were neutropenia, leukopenia, anemia, increased total bilirubin, AST elevation, injection site reaction and asthenia. Approximately 49% of NSCLC patients treated with NAVELBINE experienced at least one dose reduction due to an adverse reaction. Thirteen percent of patients discontinued NAVELBINE due to adverse reactions. The most frequent adverse reactions leading to NAVELBINE discontinuation were asthenia, dyspnea, nausea, constipation, anorexia, myasthenia and fever.
Table 1: Hematologic Adverse Reactions Experienced in > 5% of Patients Receiving NAVELBINE*†:
All patients (n=365)
(%)
NSCLC (n= 143)
(%)
Laboratory Hematologic Neutropenia < 2,000 cells/mm 3 90 80 < 500 cells/mm 3 36 29 Leukopenia < 4,000 cells/mm 3 92 81 < 1,000 cells/mm 3 15 12 Thrombocytopenia < 100,000 cells/mm 3 5 4 Anemia < 11 g/dl 83 77 < 8 g/dl 9 1 Hospitalizations due to neutropenic complications
9 8 *Grade based on modified criteria from the National Cancer Institute version 1.
†Patients with NSCLC had not received prior chemotherapy. The majority of the remaining patients had received prior chemotherapy.
Table 2: Non-hematologic Adverse Reactions Experienced in > 5% of Patients Receiving NAVELBINE*†:
All grades Grades 3+4 All Patients
(%)
NSCLC
(%)
All Patients
(%)
NSCLC
(%)
Laboratory Hepatic AST increased (n=346) 67 54 6 3 bilirubin increased (n=351) 13 9 7 5 Clinical Nausea 44 34 2 1 Asthenia 36 27 7 5 Constipation 35 29 3 2 Injection site reaction 28 38 2 5 Injection site pain 16 13 2 1 Neuropathy peripheral‡ 25 20 <2 1 Vomiting 20 15 2 1 Diarrhea 17 13 1 1 Alopecia 12 12 <1 1 Phlebitis 7 10 <1 1 Dyspnea 7 3 3 2 *Grade based on modified criteria from the National Cancer Institute version 1.
†Patients with NSCLC had not received prior chemotherapy. The majority of the remaining patients had received prior chemotherapy.
‡ Incidence of paresthesia plus hypesthesia.
Myelosuppression: In clinical trials, Grade 3-4 neutropenia, anemia, and thrombocytopenia occurred in 69%, 9% and 1%, respectively of patients receiving single-agent NAVELBINE. Neutropenia is the major dose-limiting toxicity.
Neurotoxicity: neurotoxicity was most commonly manifested as constipation, paresthesia, hypersthesia, and hyporeflexia. Grade 3 and 4 neuropathy was observed in 1% of the patients receiving single agent NAVELBINE.
Injection site reactions: Injection site reactions, including erythema, pain at injection site, and vein discoloration, occurred in approximately one third of patients; 5% were severe. Phlebitis (chemical phlebitis) along the vein proximal to the site of injection was reported in 10% of patients.
Cardiovascular toxicity: Chest pain occurred in 5% of patients; myocardial infarction occurred in less than 0.1% of patients.
Pulmonary Toxicity and Respiratory Failure: Dyspnea (shortness of breath) was reported in 3% of patients; it was severe in 2%. Interstitial pulmonary changes were documented.
Other: Hemorrhagic cystitis and the syndrome of inappropriate ADH secretion were each reported in <1% of patients.
In Combination with Cisplatin
Table 3 presents the incidence of selected adverse reactions, occurring in ≥ 10% of NAVELBINE treated patients reported in a randomized trial comparing the combination of NAVELBINE 25 mg/m 2 administered every week of each 28-day cycle and cisplatin 100 mg/m 2 administered on day 1 of each 28-day cycle versus cisplatin alone at the same dose and schedule in patients with previously untreated NSCLC (Study 1).
Patients randomized to NAVELBINE plus cisplatin received a median of 3 cycles of treatment and those randomized to cisplatin alone received a median of 2 cycles of treatment. Thirty-Five percent of the eligible patients in the combination arm required treatment discontinuation due to an adverse reaction compared to 19% in the cisplatin alone arm. The incidence of Grade 3 and 4 neutropenia was significantly higher in the NAVELBINE plus cisplatin arm (82%) compared to the cisplatin alone arm (5%). Four patients in the NAVELBINE plus cisplatin arm died of neutropenic sepsis. Seven additional deaths were reported in the combination arm: 2 from cardiac ischemia, 1 cerebrovascular accident, 1 multisystem failure due to an overdose of NAVELBINE, and 3 from febrile neutropenia.
Table 3: Adverse Reactions Experienced by > 10% of Patients on NAVELBINE plus Cisplatin versus Single-Agent Cisplatin*
NAVELBINE 25mg/m 2 plus Cisplatin 100mg/m 2 Cisplatin 100 mg/m 2 (N=212) (n=210) All Grades
(%)
Grades 3+4
(%)
All Grades
(%)
Grades 3+4
(%)
Laboratory
HematologicNeutropenia
89
82
26
5
Anemia
89
24
72
<8
Leukopenia
88
58
31
<1
Thrombocytopenia
29
5
21
<2
Febrile neutropenia †
N/A
11
N/A
0
Renal Blood creatinine increased 37
4
28
<5
ClinicalMalaise/Fatigue/Lethargy
67
12
49
8
Vomiting
60
13
60
14
Nausea
58
14
57
12
Decreased apetite
46
0
37
0
Constipation
35
3
16
1
Alopecia
34
0
14
0
Weight decreased
34
1
21
<1
Fever without infection
20
2
4
0
Hearing impaired
18
4
18
<4
Injection site reaction
17
<1
1
0
Diarrhea
17
<3
11
<2
Paraesthesia
17
<1
10
<1
Taste alterations
17
0
15
0
Peripheral numbness
11
2
7
<1
Myalgia/Arthralgia
12
<1
3
<1
Phlebitis/Thrombosis/Embolism
10
3
<1
<1
Weakness
12
<3
7
2
Infection
11
<6
<1
<1
Respiratory tract infection
10
<5
3
3
*Graded according to the standard SWOG criteria version 1.
†Categorical toxicity grade not specified
Table 4 presents the incidence of selected adverse reactions, occurring in ≥ 10% of NAVELBINE treated patients reported in a randomized trial of NAVELBINE plus cisplatin, vindesine plus cisplatin and NAVELBINE as a single agent in patients with stage III or IV NSCLC who had not received prior chemotherapy. A total of 604 patients received either NAVELBINE 30 mg/m 2 every week plus cisplatin 120 mg/m 2 on Day 1 and Day 29, then every 6 weeks thereafter (N=207), vindesine 3 mg/m 2 for 6 weeks, then every other week thereafter plus cisplatin 120 mg/m 2 on Days 1 and Day 29, then every 6 weeks thereafter (N=193) or NAVELBINE 30mg/m 2 every week (N=204).
Patients randomized to NAVELBINE plus cisplatin received a median of 15 weeks of treatment, vindesine plus cisplatin 12 weeks and NAVELBINE received 13 weeks. Grade 3 and 4 neutropenia was significantly greater in the NAVELBINE plus cisplatin arm (78%) compared to vindesine plus cisplatin (48%) and NAVELBINE as a single agent (53%). Neurotoxicity, including peripheral neuropathy and constipation, was reported in 44% (Grade 3-4, 7%) of the patients receiving NAVELBINE plus cisplatin, 58% (Grade 3-4, 17%) of the patients receiving vindesine and cisplatin and 44% (Grade 3-4, 8.5%) of the patients receiving NAVELBINE as a single agent.
Study discontinuation due to an adverse reaction was required in 27, 22 and 10% of the patients randomized to NAVELBINE plus cisplatin, vindesine plus cisplatin and cisplatin alone arms, respectively.
Table 4: Adverse Reactions Experienced by > 10 % of Patients from a Comparative Trial of NAVELBINE Plus Cisplatin versus Vindesine Plus Cisplatin versus Single Agent NAVELBINE*
NAVELBINE/Cisplatin† Vindesine/Cisplatin† NAVELBINE§ All Grades
(%)
Grades 3+4
(%)
All Grades
(%)
Grades 3+4
(%)
All Grades
(%)
Grades 3+4
(%)
Laboratory Hematologic Neutropenia 95 78 79 48 85 53 Leukopenia 94 57 82 27 83 32 Thrombocytopenia 15 4 10 3.5 3 0 Renal Blood creatinine increased ¦ 46 N/A 37 N/A 13 N/A
ClinicalNausea/Vomiting 74 30 72 25 31 2 Alopecia 51 7.5 56 14 30 2 Neurotoxicity ¶ 44 7 58 17 44 8.5 Diarrhea 25 1.5 24 1 12 0.5 Injection site reaction 17 2.5 7 0 22 2 Ototoxicity 10 2 14 1 1 0 * Grade based on criteria from the World Health Organization (WHO).
† n=194 to 207; all patients receiving NAVELBINE/cisplatin with laboratory and non-laboratory data.
‡ n=173 to 192; all patients receiving vindesine/cisplatin with laboratory and non-laboratory data.
§ n=165 to 201; all patients receiving NAVELBINE with laboratory and non-laboratory data.
¦ Categorical toxicity grade not specified.
¶ Neurotoxicity includes peripheral neuropathy and constipation.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of NAVELBINE. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Infections and infestations: pneumonia
Immune system disorders: anaphylactic reaction, pruritus, urticaria, angioedema
Nervous system disorders: loss of deep tendon reflexes, muscular weakness, gait disturbance, headache
Ear and labyrinth disorders: vestibular disorder, hearing impaired
Cardiac disorders: tachycardia
Respiratory disorders: pulmonary edema
Vascular disorders: pulmonary embolism, deep vein thrombosis, hypertension, hypotension, flushing, vasodilatation
Gastrointestinal disorders: mucosal inflammation, dysphagia, pancreatitis
Skin disorders: generalized cutaneous reactions (rash), palmar-plantar erythrodysesthesia syndrome
Musculoskeletal and connective tissue disorders: jaw pain, myalgia, arthralgia
General disorders and administration site conditions: injection site rash, urticaria, blistering, sloughing of skin
Injury, poisoning and procedural complications: radiation recall phenomenon, dermatitis, esophagitis
Laboratory abnormalities: electrolyte imbalance including hyponatremia
Other: tumor pain, back pain, abdominal pain
- 7 DRUG INTERACTIONS
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Based on findings from animal studies and its mechanism of action [see Clinical Pharmacology (12.1))], NAVELBINE can cause fetal harm when administered to a pregnant woman. Available human data are insufficient to inform the drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction studies in mice and rabbits, embryo and fetal toxicity were observed with administration of vinorelbine at doses approximately 0.33 and 0.18 times the human therapeutic dose, respectively ( see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies are 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
In a mouse embryofetal development study, administration of a single dose of vinorelbine at a dose level of 9 mg/m2 or greater (approximately 0.33 times the recommended human dose based on body surface area) was embryotoxic and fetotoxic. Vinorelbine was embryotoxic and fetotoxic to pregnant rabbits when administered every 6 days during the period of organogenesis at doses of 5.5 mg/m2 (approximately 0.18 times the recommended human dose based on body surface area) or greater. At doses that did not cause maternal toxicity in either species, vinorelbine administration resulted in reduced fetal weight and delayed ossification.
8.2 Lactation
Risk Summary
There are no data on the presence of vinorelbine in human milk or its effects on the breastfed infant or on milk production. Because of the potential for serious adverse reactions in breastfed infants from vinorelbine, advise women not to breastfeed during treatment with NAVELBINE and for 9 days after the final dose.
8.3 Females and Males of Reproductive Potential
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating NAVELBINE [see Use in Specific Populations (8.1)].
Contraception
Females
NAVELBINE can cause fetal harm when administered to pregnant women [see Use in Specific Populations (8.1)]. Advise female patients of reproductive potential to use effective contraception during treatment with NAVELBINE and for 6 months after the final dose.
Males
NAVELBINE may damage spermatozoa [see Nonclinical Toxicology (13.1)]. Advise males with female sexual partners of reproductive potential to use effective contraception during treatment with NAVELBINE and for 3 months after the final dose.
Infertility
Males
Based on animal findings, NAVELBINE may impair fertility in males [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and effectiveness of NAVELBINE in pediatric patients have not been established. Results from a single-arm study of NAVELBINE administered at the dose of 33.75 mg/m 2 (for 35 patients) or at the dose of 30mg/m 2 (for 11 patients) every week during 6 weeks followed by 2 weeks of rest was evaluated (courses of 8 weeks).Forty-six patients age 1 to 25 (median 11 years) with recurrent solid malignant tumors, including rhabdomyosarcoma or undifferentiated sarcoma (N=21 patients), neuroblastoma (N= 4 patients), and central nervous system (CNS) tumors (N=21 patients) were enrolled. The most significant grade 3 or 4 hematological adverse reactions were neutropenia (70%) and anemia (33%). The most significant grade 3 or 4 non-hematological toxicity adverse reactions were motor (15%) or cranial (13%) neuropathy, hypoxia (13%) and dyspnea (11%). Objective tumor response was observed in 2 out of 21 patients with rhabdomyosarcoma or undifferentiated sarcoma. No objective tumor response was observed in patients with CNS tumors (N=21) or neuroblastoma (N=4).
8.5 Geriatric Use
Of the 769 number of patients who received NAVELBINE alone and NAVELBINE in combination with Cisplatin in studies 1, 2 and 3, 247 patients were 65 years of age or older. No overall differences in safety, efficacy and pharmacokinetic parameters were observed between these patients and younger patients. [see Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
The influence of hepatic impairment on the pharmacokinetics of NAVELBINE has not been evaluated, but the liver plays an important role in the metabolism of NAVELBINE. Elevations of aspartate aminotransferase occur in > 60% of the patients receiving NAVELBINE alone (6% Grade 3-4). Therefore, exercise caution in patients with hepatic impairment. Reduce the dose of NAVELBINE for patients with elevated serum total bilirubin concentrations [see Dosage and Administration (2.2) and Warnings and Precautions (5.2)].
-
10 OVERDOSAGE
There is no known antidote for overdoses of NAVELBINE. Overdoses involving quantities up to 10 times the recommended dose (30 mg/m 2) have been reported. The toxicities described were consistent with those listed in the ADVERSE REACTIONS section including paralytic ileus, stomatitis, and esophagitis. Bone marrow aplasia, sepsis, and paresis have also been reported. Fatalities have occurred following overdose of NAVELBINE. If overdosage occurs, general supportive measures together with appropriate blood transfusions, growth factors, and antibiotics should be instituted as deemed necessary by the physician.
-
11 DESCRIPTION
NAVELBINE (vinorelbine) Injection contains vinorelbine, a semi-synthetic vinca alkaloid.
The molecular formula for vinorelbine tartrate, USP is C 45H 54N 4O 8●2C 4H 6O 6.
It has a molecular weight of 1079.11.
The structural formula is as follows:
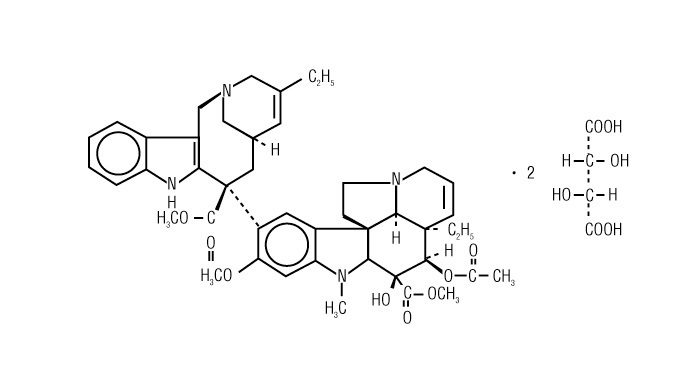
Vinorelbine tartrate, is a white to light yellow amorphous powder. It is easily soluble in water, very soluble in methanol, and practically insoluble in hexane.
NAVELBINE Injection is a sterile nonpyrogenic aqueous solution. Each milliliter of solution contains 13.85 mg vinorelbine tartrate USP equivalent to 10 mg vinorelbine in Water for Injection, USP. The pH of NAVELBINE Injection is approximately 3.5.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Vinorelbine is a vinca alkaloid that interferes with microtubule assembly. The antitumor activity of vinorelbine is thought to be due primarily to inhibition of mitosis at metaphase through its interaction with tubulin. Vinorelbine may also interfere with: 1) amino acid, cyclic AMP, and glutathione metabolism, 2) calmodulin-dependent Ca++-transport ATPase activity, 3) cellular respiration, and 4) nucleic acid and lipid biosynthesis. Vinorelbine inhibited mitotic microtubule formation in intact mouse embryo tectal plates at a concentration of 2 μM inducing a blockade of cells at metaphase, but produced depolymerization of axonal microtubules at a concentration
40 μM, suggesting a modest selectivity of vinorelbine for mitotic microtubules.
12.3 Pharmacokinetics
The pharmacokinetics of vinorelbine were studied in 49 patients who received doses of 30 mg/m 2 administered as 15- to 20-minute constant-rate infusions. Vinorelbine concentrations in plasma decay in a triphasic manner.
Distribution
Steady-state volume of distribution (V SS) values range from 25.4 to 40.1 L/kg. Vinorelbine demonstrated high binding to human platelets and lymphocytes. The free fraction was approximately 0.11 in human plasma over a concentration range of 234 to 1169 ng/mL. The binding to plasma constituents in cancer patients ranged from 79.6% to 91.2%. Vinorelbine binding was not altered in the presence of cisplatin, 5-fluorouracil, or doxorubicin.
Elimination
The terminal phase half-life averages 27.7 to 43.6 hours and the mean plasma clearance ranges from 0.97 to 1.26 L/hr/kg.
Metabolism
Vinorelbine undergoes substantial hepatic elimination in humans, with large amounts recovered in feces. Two metabolites of vinorelbine have been identified in human blood, plasma, and urine; vinorelbine N-oxide and deacetylvinorelbine. Deacetylvinorelbine has been demonstrated to be the primary metabolite of vinorelbine in humans, and has been shown to possess antitumor activity similar to vinorelbine. Therapeutic doses of vinorelbine (30 mg/m 2) yield very small, if any, quantifiable levels of either metabolite in blood or urine. The metabolism of vinorelbine is mediated by hepatic cytochrome P450 isoenzymes in the CYP3A subfamily.
Excretion
After intravenous administration of radioactive vinorelbine, approximately 18% and 46% of administered radioactivity was recovered in urine and feces, respectively. In a different study, 10.9% + 0.7% of a 30-mg/m 2 intravenous dose was excreted as parent drug in urine.
Specific Populations
Elderly: Age has no effect on the pharmacokinetics (CL, V SS and t 1/2) of vinorelbine.
Drug Interactions
The pharmacokinetics of vinorelbine are not influenced by the concurrent administration of cisplatin.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of NAVELBINE has not been studied. Vinorelbine has been shown to affect chromosome number and possibly structure in vivo (polyploidy in bone marrow cells from Chinese hamsters and a positive micronucleus test in mice). It was not mutagenic in the Ames test and gave inconclusive results in the mouse lymphoma TK Locus assay.
Vinorelbine did not affect fertility to a statistically significant extent when administered to rats on either a once-weekly (9 mg/m 2, approximately one third the human dose) or alternate-day schedule (4.2 mg/m 2, approximately 0.14 times the human recommended dose) prior to and during mating. In male rats, administration of vinorelbine twice weekly for 13 or 26 weeks at dose levels of 2.1 and 7.2 mg/m 2 (approximately 0.07 and 0.24 times the recommended human dose), respectively, resulted in decreased spermatogenesis and prostate/seminal vesicle secretion.
-
14 CLINICAL STUDIES
14.1 Combination Use with Cisplatin
The safety and efficacy of NAVELBINE in combination with cisplatin was evaluated in two randomized, multicenter trials.
Cisplatin 100mg/m2
Study 1 was a randomized, multicenter, open-label trial of NAVELBINE plus cisplatin and cisplatin alone for the treatment of stage IV or stage IIIb NSCLC patients with malignant pleural effusion or multiple lesions in more than one lobe of the ipsilateral lung who had not received prior chemotherapy. A total of 432 patients were randomized 1:1 to receive either NAVELBINE 25 mg/m 2 on Day 1 then every week of each 28-day cycle with cisplatin 100 mg/m 2 administered on Day 1 of each 28-day cycle (N=214) or cisplatin 100 mg/m 2 on Day 1 of each 28-day cycle (N=218).
Patient demographics and disease characteristics were similar between arms. Of the overall study population, the median age was 64 (range 33-84), 66% were male, 80% were Caucasian, 92% had stage IV disease and 8% stage IIIB, 53% had adenocarcinoma, 21% squamous cell, 14% large cell histology. The major efficacy outcome measure was overall survival. The efficacy results are presented in Table 7 and Figure 1.
Table 7. Efficacy Results (Study 1)
NAVELBINE plus Cisplatin Cisplatin (N=214) (N=218) Overall Survival Median Survival in months (95% CI) 7.8 (6.9, 9.6 ) 6.2 (5.4, 7.7) Unstratified log-rank p-value 0.01 Overall Response rate (ORR)
Evaluable patients
ORR (95% CI)
N = 206
19% (14%, 25%)
N=209
8% (5%, 13% )Chi-square test p-value <0.001 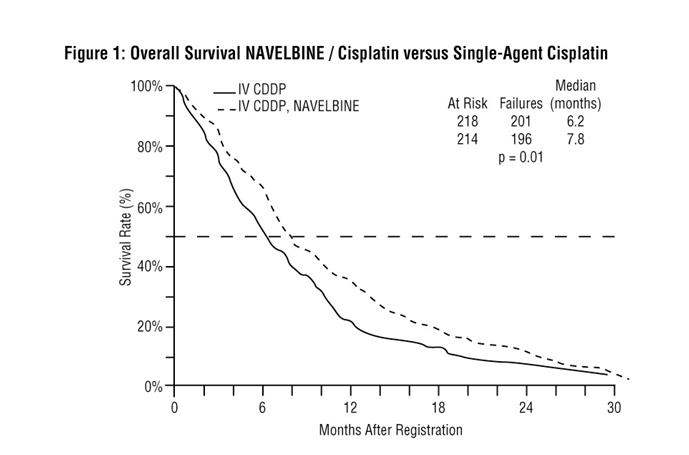
Cisplatin 120mg/m2
Study 2 was a randomized, 3-arm, open-label, multicenter trial of NAVELBINE plus cisplatin, vindesine plus cisplatin and NAVELBINE as a single agent for the treatment of patients with stage III or IV NSCLC who had not received prior chemotherapy. The study was conducted in Europe. A total of 612 patients were randomized 1:1:1 to receive NAVELBINE 30 mg/m 2 every week of a 6-week cycle plus cisplatin 120 mg/m 2 on Day 1 and Day 29, then every 6 weeks thereafter (N=206); and vindesine 3 mg/m 2 for 6 weeks, then every other week thereafter plus cisplatin 120 mg/m 2 on Days 1 and Day 29, then every 6 weeks thereafter (N=200) or NAVELBINE 30mg/m 2 every week of a 6-week cycle (N=206). The main efficacy outcome measure was to compare overall survival between NAVELBINE plus cisplatin and vindesine plus cisplatin. The other efficacy outcome measure was to compare overall survival in the better of the two combination regimens to that of NAVELBINE single agent.
Patient demographics were in general similar between arms: the median age of the overall population was 60 years (range 30 to 75), 90% were male, 78% had WHO performance status of 0 or 1. Tumor characteristics were in general similar with the exception of histologic subtype of NSCLC. Adenocarcinoma was the histologic subtype in 32% of patients in the NAVELBINE plus cisplatin arm, 40% of patients in vindesine plus cisplatin arm and 28% of patients on the NAVELBINE arm. Ten percent of the patients had stage IIIA disease, 28% stage IIIB and 50% stage IV. Twelve percent of the patients had received prior surgery or radiotherapy.
The efficacy results of Study 2 are presented in Table 8.
Table 8. Efficacy Results (Study 2)
NAVELBINE NAVELBINE plus Vindesine plus (N=206) cisplatin (N=206) cisplatin (N=200) Median survival in 7.2 (5.4-9.1) 9.2 (7.4-11.1) 7.4 (6.1-9.1) months (99.5% CI) Unstratified log-rank n/a 1 0.087 p-value 0.05 n/a Overall Response (ORR) Evaluable Patients N=205 N=203 N=198 ORR (95% CI) 14% (10%, 20%) 28% (22%, 35%) 19% (14%, 25% ) Chi-square test p-value n/a 0.03 < 0.001 n/a 1n/a = not applicable
14.2 Single Agent
The safety and efficacy of NAVELBINE as a single agent was evaluated in one randomized multi-center trial.
Study 3 was a randomized, open-label clinical trial of NAVELBINE or fluorouracil (FU) plus leucovorin (LV) in patients with Stage IV NSCLC who had not received prior chemotherapy A total of 211 patients were randomized 2:1 to receive NAVELBINE 30 mg/m 2 weekly of an 8-week cycle (N=143) or FU 425 mg/m 2 bolus intravenously plus LV 20 mg/m 2 bolus intravenously daily for 5 days of a 4-weeks cycle (N=68).
Patient demographics and disease characteristics were in general similar between arms. In the overall population, the median age was 61 years (range 32 - 83), 74% were male, 88% were White, 46% had adenocarcinoma histology. Fifty percent of the patients had Karnofsky performance status ≥ 90 in the NAVELBINE arm compared to 38% in the FU and LV arm.
The primary efficacy outcome of the study was overall survival. The median survival time was 30 weeks versus 22 weeks for patients receiving NAVELBINE versus FU/LV, respectively (P=0.06). Partial objective responses were observed in 11.1% (95% CI=6.2%, 17.9%) and 3.5% (95% CI=0.4%, 11.9%) of patients who received NAVELBINE and FU/LV, respectively.
- 15 REFERENCES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
NAVELBINE Injection is a clear, colorless to pale yellow aqueous solution available in single-dose vials with royal blue caps, individually packaged in a carton as:
- 10 mg/1 mL (NDC: 64370-532-01).
- 50 mg/5 mL (NDC: 64370-532-02).
Store the vials at 2° to 8°C (36° to 46°F) in the carton. Protect from light. DO NOT FREEZE. Unopened vials of NAVELBINE are stable at 25°C (77°F) for up to 72 hours. Store diluted solutions of NAVELBINE at 5°F to 30°C (41° to 86°F) [ see Dosage and Administration (2.3)].
NAVELBINE is a cytotoxic drug. Follow applicable special handling and disposal procedures. 1
-
17 PATIENT COUNSELING INFORMATION
Inform patients of the following:
- Myelosuppression
Advise patients to contact a healthcare provider for new onset fever, or symptoms of infection [see Warnings and Precautions (5.1)].
- Constipation and bowel obstruction
Advise patients to follow a diet rich in fibers, drink fluids to stay well hydrated and use stool softeners to avoid constipation. Contact a health care provider for severe constipation, new onset abdominal pain, nausea and vomiting [see Warnings and Precautions (5.3)].
- Neurologic toxicity
Advise patients to contact a health care provider for new onset or worsening of numbness, tingling, decrease sensation or muscle weakness [see Warnings and Precautions (5.5)].
- Pulmonary Toxicity
Advise patients to contact a healthcare provider for new onset or worsening of shortness of breath, cough, wheezing or other new pulmonary symptoms [see Warnings and Precautions (5.6)].
- Embryo-Fetal Toxicity
Advise pregnant women of the potential risk to a fetus. Advise females to inform their healthcare provider of a known or suspected pregnancy [see Warning and Precautions (5.7) and Use in Specific Populations (8.1, 8.3)].
Advise females of reproductive potential to use effective contraception during treatment with NAVELBINE and for 6 months after the final dose [see Use in Specific Populations (8.3)].
Advise males with female partners of reproductive potential to use effective contraception during treatment with NAVELBINE and for 3 months after the final dose [see Use in Specific Population (8.7) and Nonclinical Toxicology (13.1)].- Lactation
Advise women not to breastfeed during treatment with NAVELBINE and for 9 days after the final dose [see Use in Specific Populations (8.2)].
- Infertility
Advise males of reproductive potential that NAVELBINE may impair fertility [see Use in Specific Populations (8.3)].

Manufactured by:
Pierre Fabre Médicament
45 place Abel Gance - 92100 Boulogne - FRANCE
Distributed by:
Pierre Fabre Pharmaceuticals, Inc.
Parsippany, NJ 07054
Made in France
-
PRINCIPAL DISPLAY PANEL - 10 MG/ML VIAL CARTON
NDC: 64370-532-01
1 mL Single-Use Vial
NAVELBINE
(VINORELBINE) INJECTION
10 mg/mL
Rx only
Caution: Cytotoxic Agent
FOR INTRAVENOUSE USE ONLY.
MUST BE DILUTED
FOR ADMINISTRATION.
-
PRINCIPAL DISPLAY PANEL - 50 MG/ML VIAL CARTON
NDC: 64370-532-02
5 mL Single-Use Vial
NAVELBINE
(VINORELBINE) INJECTION
50 mg/mL
(10 mg/mL)
Rx only
Caution: Cytotoxic Agent
FOR INTRAVENOUSE USE
ONLY.
MUST BE DILUTED
FOR ADMINISTRATION.
-
INGREDIENTS AND APPEARANCE
NAVELBINE
vinorelbine tartrate injectionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 64370-532 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength VINORELBINE TARTRATE (UNII: 253GQW851Q) (VINORELBINE - UNII:Q6C979R91Y) VINORELBINE 10 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64370-532-01 1 in 1 CARTON 11/15/2005 1 1 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product 2 NDC: 64370-532-02 1 in 1 CARTON 11/15/2005 2 5 mL in 1 VIAL, SINGLE-USE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA020388 11/15/2005 10/31/2021 Labeler - Pierre Fabre Pharmaceuticals, Inc. (968997101) Registrant - Pierre Fabre Pharmaceuticals, Inc. (968997101) Establishment Name Address ID/FEI Business Operations Pierre Fabre Medicament 267116254 analysis(64370-532) , api manufacture(64370-532) Establishment Name Address ID/FEI Business Operations Pierre Fabre Medicament Production 504638276 analysis(64370-532) , manufacture(64370-532)
Trademark Results [NAVELBINE]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NAVELBINE 73632692 1452870 Live/Registered |
PIERRE FABRE MEDICAMENT 1986-11-28 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.