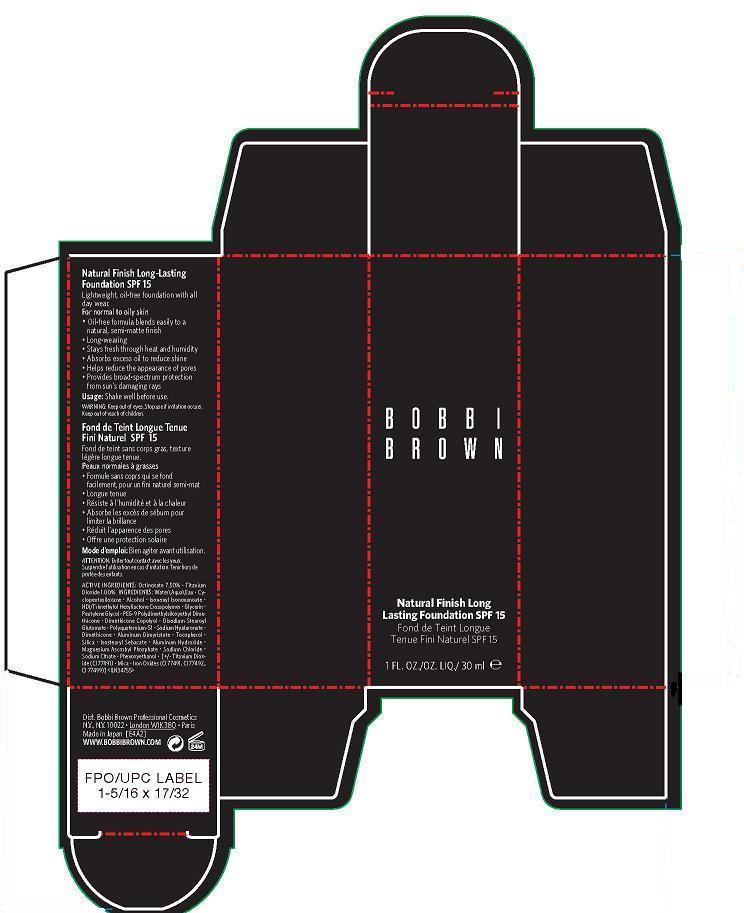
NATURAL FINISH LONG LASTING FOUNDATION SPF 15- octinoxate, titanium dioxide cream
NATURAL FINISH by
Drug Labeling and Warnings
NATURAL FINISH by is a Otc medication manufactured, distributed, or labeled by Bobbi Brown Professional Cosmetics Inc., ESTEE LAUDER N.V., Len-Ron Manufacturing Division of Aramis Inc., Aramis Inc., Northtec Bristol, Northtec Keystone, PADC 1, Estee Lauder Pennsylvania Distribution Center 2, Estee Lauder Cosmetics, Ltd., Estee Lauder Cosmetics, Ltd, Estee Lauder Cosmetics Distribution Center, Estee Lauder Kabushiki Kaisha, Whitman Laboratories Ltd., Aveda Corporation, J. O. COSMETICS. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- WHEN USING
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
NATURAL FINISH LONG LASTING FOUNDATION SPF 15
octinoxate, titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 64141-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 mL in 100 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 1.0 mL in 100 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 64141-002-01 1 in 1 CARTON 1 NDC: 64141-002-02 30 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 10/01/2007 Labeler - Bobbi Brown Professional Cosmetics Inc. (627131279) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 manufacture Establishment Name Address ID/FEI Business Operations Len-Ron Manufacturing Division of Aramis Inc. 809771152 manufacture Establishment Name Address ID/FEI Business Operations Aramis Inc. 042918826 manufacture Establishment Name Address ID/FEI Business Operations Northtec Bristol 949264774 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Northtec Keystone 618107429 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations PADC 1 110482184 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Estee Lauder Pennsylvania Distribution Center 2 828534516 manufacture, relabel, repack Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics, Ltd. 255175580 manufacture Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics, Ltd 253616536 manufacture Establishment Name Address ID/FEI Business Operations Estee Lauder Cosmetics Distribution Center 208579636 manufacture, label, relabel Establishment Name Address ID/FEI Business Operations Estee Lauder Kabushiki Kaisha 712808195 relabel, repack Establishment Name Address ID/FEI Business Operations Whitman Laboratories Ltd. 216866277 manufacture Establishment Name Address ID/FEI Business Operations Aveda Corporation 071352058 manufacture Establishment Name Address ID/FEI Business Operations J. O. COSMETICS 692780372 manufacture
Trademark Results [NATURAL FINISH]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 NATURAL FINISH 85096691 not registered Dead/Abandoned |
Amarte USA Holdings, Inc. 2010-07-30 |
 NATURAL FINISH 78268533 not registered Dead/Abandoned |
Dee Cee, Inc. 2003-06-30 |
 NATURAL FINISH 77266151 not registered Dead/Abandoned |
Beauty Holding LLC 2007-08-28 |
 NATURAL FINISH 77085674 not registered Dead/Abandoned |
Industrias Auxiliares Faus, S.L. 2007-01-18 |
 NATURAL FINISH 75125451 2111407 Dead/Cancelled |
MATRIX ESSENTIALS, INC. 1996-06-25 |
 NATURAL FINISH 72323758 0892249 Dead/Expired |
CHESEBROUGH-POND'S INC. 1969-04-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.