WINCO MOISTURE SPF 15 LIP BALM- oxybenzone, octinoxate, petrolatum stick
Winco Moisture SPF 15 Lip Balm by
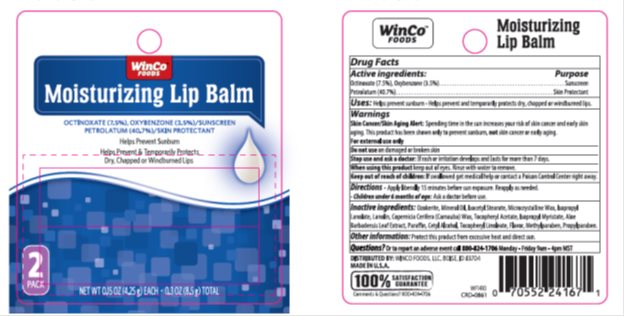
Drug Labeling and Warnings
Winco Moisture SPF 15 Lip Balm by is a Otc medication manufactured, distributed, or labeled by Winco, OraLabs. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Uses
-
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early aging.
For external use only
Do not use on broken or damaged skin
Stop use and ask a doctor: If rash or irritation develops and lasts for more than 7 days.
When using this product keep out of eyes. Rinse with water to remove.
- Directions
- Inactive Ingredients
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
WINCO MOISTURE SPF 15 LIP BALM
oxybenzone, octinoxate, petrolatum stickProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 67091-250 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 7.5 mg in 1 g OXYBENZONE (UNII: 95OOS7VE0Y) (OXYBENZONE - UNII:95OOS7VE0Y) OXYBENZONE 3.5 mg in 1 g PETROLATUM (UNII: 4T6H12BN9U) (PETROLATUM - UNII:4T6H12BN9U) PETROLATUM 40.7 mg in 1 g Inactive Ingredients Ingredient Name Strength CERESIN (UNII: Q1LS2UJO3A) 17.13 mg in 1 g LIGHT MINERAL OIL (UNII: N6K5787QVP) 16.75 mg in 1 g ISOCETYL STEARATE (UNII: 3RJ7186O9W) 3.50 mg in 1 g MICROCRYSTALLINE WAX (UNII: XOF597Q3KY) 3.00 mg in 1 g LANOLIN (UNII: 7EV65EAW6H) 1 mg in 1 g .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) .60 mg in 1 g Product Characteristics Color WHITE Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 67091-250-30 1 g in 1 CONTAINER; Type 0: Not a Combination Product 01/02/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 01/02/2015 Labeler - Winco (056098817) Registrant - OraLabs (801824756) Establishment Name Address ID/FEI Business Operations OraLabs 801824756 MANUFACTURE(67091-250) , LABEL(67091-250)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.