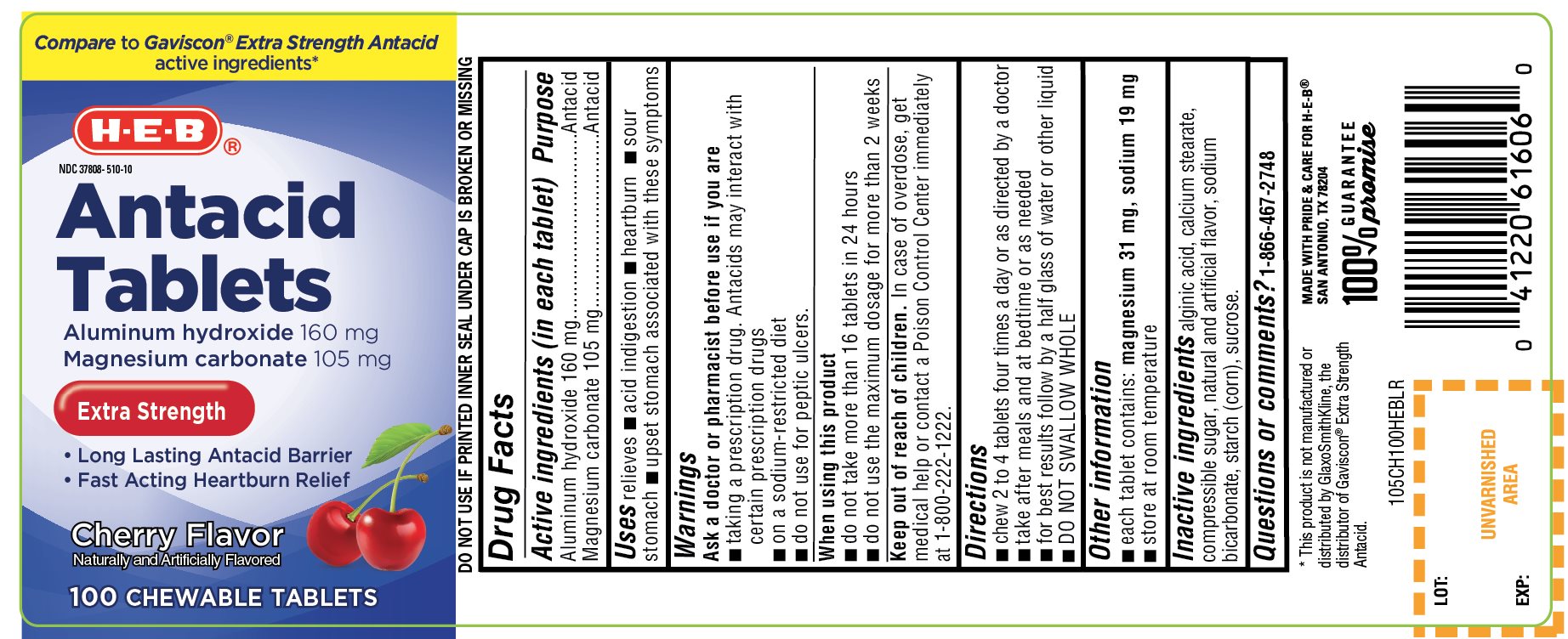
HEB EXTRA STRENGTH CHERRY FLAVOR- aluminum hydroxide and magnesium carbonate tablet, chewable
HEB Extra Strength by
Drug Labeling and Warnings
HEB Extra Strength by is a Otc medication manufactured, distributed, or labeled by HEB. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredients (in each tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor or pharmacist before use if you are
- ▪ taking a prescription drug. Antacids may interact with certain prescription drugs
- ▪ on a sodium-restricted diet
- ▪ do not use for peptic ulcers.
- Directions
- Other information
- Inactive ingredients
-
Principal Display Panel
HEB®
NDC: 37808-510-10
Antacid Tablets
Compare To Gaviscon®Extra Strength active ingredients*
EXTRA STRENGTH
Aluminum Hydroxide, 160 mg
Magnesium Carbonate, 105 mg
- Long Lasting Antacid Barrier
- Fast-Acting Heartburn Relief
Cherry Flavor
Naturally and Artificially Flavored
100 CHEWABLE TABLETS
MADE WITH PRIDE & CARE FOR H-E-B
SAN ANTONIO, TX 78204
100% GUARANTEE Promise
DO NOT USE IF PRINTED INNER SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by GlaxoSmithKline, the distributor of Gaviscon® Extra Strength Antacid.
-
INGREDIENTS AND APPEARANCE
HEB EXTRA STRENGTH CHERRY FLAVOR
aluminum hydroxide and magnesium carbonate tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 37808-510 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALUMINUM HYDROXIDE (UNII: 5QB0T2IUN0) (ALUMINUM HYDROXIDE - UNII:5QB0T2IUN0) ALUMINUM HYDROXIDE 160 mg MAGNESIUM CARBONATE (UNII: 0E53J927NA) (CARBONATE ION - UNII:7UJQ5OPE7D) MAGNESIUM CARBONATE 105 mg Inactive Ingredients Ingredient Name Strength ALGINIC ACID (UNII: 8C3Z4148WZ) CALCIUM STEARATE (UNII: 776XM7047L) CORN SYRUP (UNII: 9G5L16BK6N) SODIUM BICARBONATE (UNII: 8MDF5V39QO) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) Product Characteristics Color WHITE Score no score Shape ROUND Size 17mm Flavor CHERRY Imprint Code RP105 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 37808-510-10 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/20/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part331 03/20/2019 Labeler - HEB (007924756)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.