2023-10-09 De-listing 74472-003_SOME BY MI AHA BHA PHA 30 DAYS MIRACLE TONER
SOME BY MI AHA BHA PHA 30 DAYS MIRACLE TONER by
Drug Labeling and Warnings
SOME BY MI AHA BHA PHA 30 DAYS MIRACLE TONER by is a Otc medication manufactured, distributed, or labeled by PERENNEBELL Co., Ltd., reBom Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
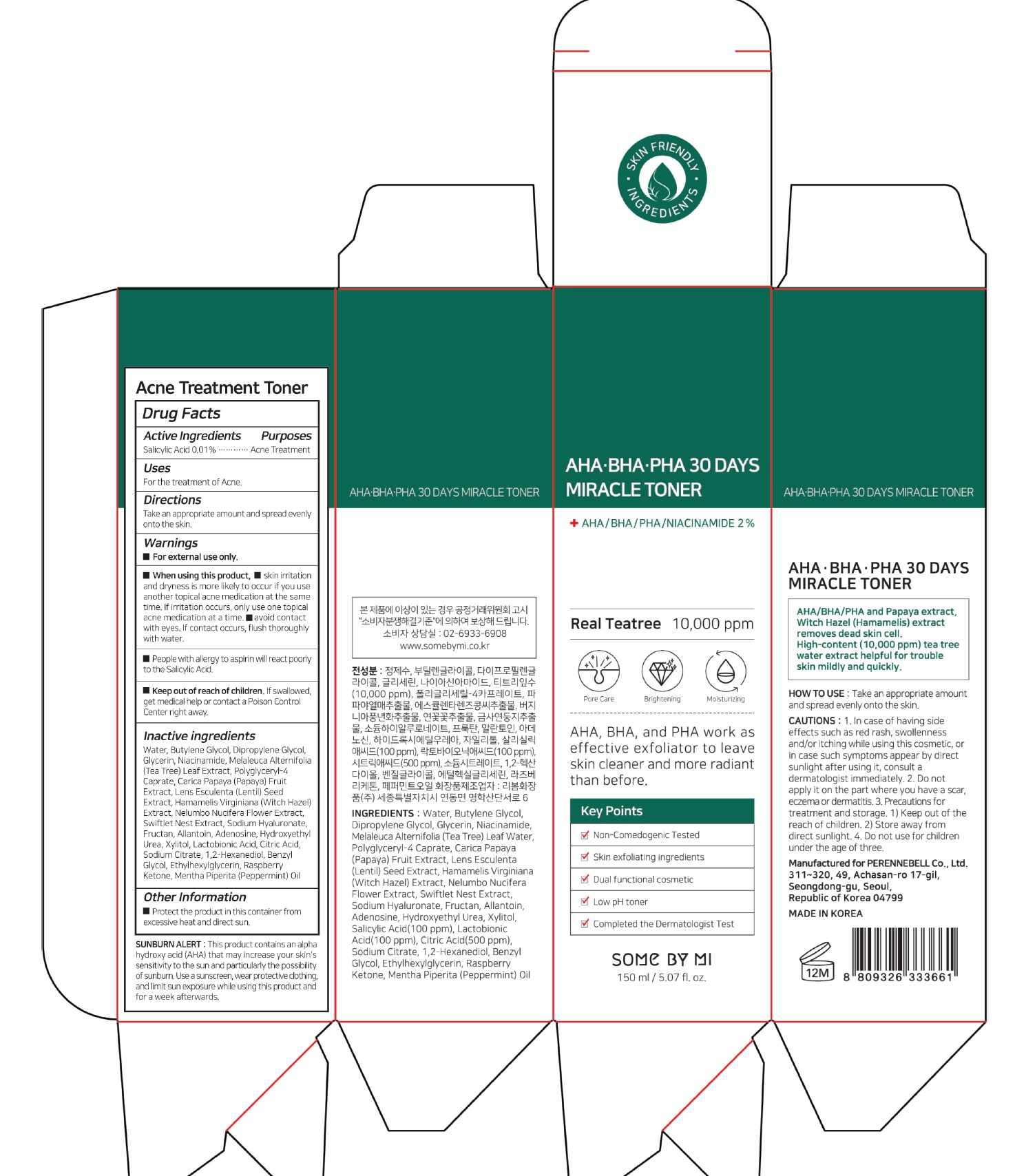
SOME BY MI AHA BHA PHA 30 DAYS MIRACLE TONER- salicylic acid liquid
PERENNEBELL Co., Ltd.
----------
2023-10-09 De-listing 74472-003_SOME BY MI AHA BHA PHA 30 DAYS MIRACLE TONER
For external use only
When using this product, skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, only use one topical acne medication at a time. avoid contact with eyes. If contact occurs, flush thoroughly with water.
People with allergy to aspirin will react poorly to the Salicylic Acid.
Keep out of reach of the children. If product is swallowed, get medical help or contact a poison control center right away.
Water, Butylene Glycol, Dipropylene Glycol, Glycerin, Niacinamide, Melaleuca Alternifolia (Tea Tree) Leaf Extract, Polyglyceryl-4 Caprate, Carica Papaya (Papaya) Fruit Extract, Lens Esculenta (Lentil) Seed Extract, Hamamelis Virginiana (Witch Hazel) Extract, Nelumbo Nucifera Flower Extract, Swiftlet Nest Extract, Sodium Hyaluronate, Fructan, Allantoin, Adenosine, Hydroxyethyl Urea, Xylitol, Lactobionic Acid, Citric Acid, Sodium Citrate, 1,2-Hexanediol, Benzyl Glycol, Ethylhexylglycerin, Raspberry Ketone, Mentha Piperita (Peppermint) Oil
| SOME BY MI AHA BHA PHA 30 DAYS MIRACLE TONER
salicylic acid liquid |
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - PERENNEBELL Co., Ltd. (694788814) |
| Registrant - PERENNEBELL Co., Ltd. (694788814) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| reBom Co., Ltd. | 688733595 | manufacture(74472-003) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.