Vetmedin® ChewVetmedin Chew 1.25 mg chewable tablets for dogsVetmedin Chew 5 mg chewable tablets for dogsVetmedin Chew 10 mg chewable tablets for dogsPimobendan
Vetmedin by
Drug Labeling and Warnings
Vetmedin by is a Animal medication manufactured, distributed, or labeled by Boehringer Ingelheim Animal Health USA Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VETMEDIN CHEW- pimobendan tablet, chewable
Boehringer Ingelheim Animal Health USA Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
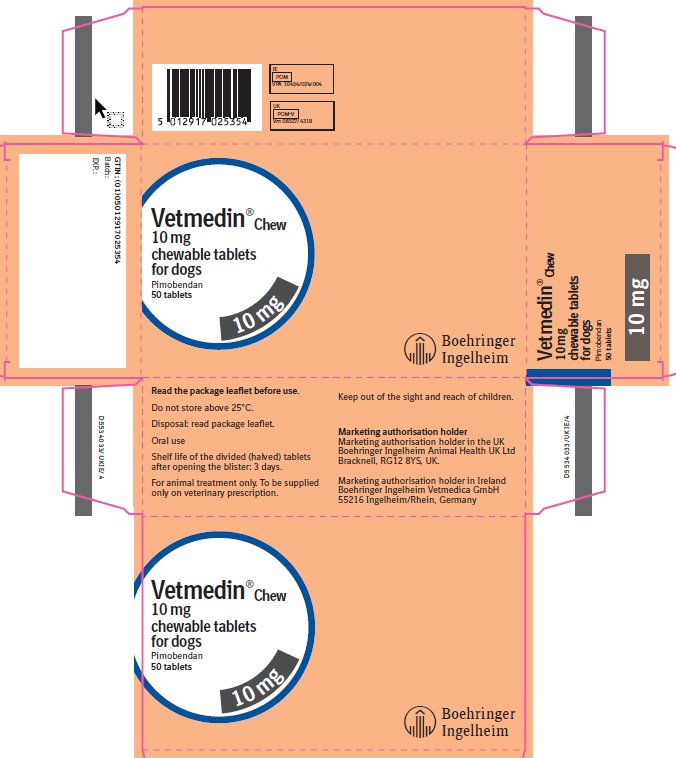
Vetmedin® Chew
Vetmedin Chew 1.25 mg chewable tablets for dogs
Vetmedin Chew 5 mg chewable tablets for dogs
Vetmedin Chew 10 mg chewable tablets for dogs
Pimobendan
Client Information Sheet
Client Information Sheet for Vetmedin® Chew (pimobendan)
Replacement product for Vetmedin® (pimobendan) chewable tablets
Contact your Veterinarian BEFORE administering this product to your dog.
This Client Information Sheet contains important information about VETMEDIN CHEW, a product used as a temporary replacement for US FDA-approved VETMEDIN chewable tablets.
VETMEDIN CHEW is authorized for marketing in the United Kingdom and Ireland to treat congestive heart failure in dogs. You should read this information before you start giving VETMEDIN CHEW and review it each time the prescription is refilled as there may be new information.
This information sheet is provided only as a summary and does not take the place of instructions from your veterinarian. If you have received this product from a pharmacy and not directly from your veterinarian, you should contact your veterinarian to inform them that you have received this replacement product. Talk with your veterinarian if you do not understand any of this information or if you want to know more about VETMEDIN CHEW.
Note to prescribing veterinarians
The dose administered should be as stated in the FDA approved VETMEDIN chewable tablets package insert, accessible at: /DailyMed/5441e5b2-cdc0-477a-bd9a-ec5f8d912281
Why did I receive VETMEDIN CHEW?
Because of a shortage in the supply of the FDA-approved VETMEDIN chewable tablets, the FDA is allowing temporary use of VETMEDIN CHEW in the United States.
Is VETMEDIN CHEW the same as VETMEDIN chewable tablets?
- The active ingredient in VETMEDIN CHEW is pimobendan, just like in Vetmedin chewable tablets, the US-approved product.
- Both VETMEDIN CHEW and VETMEDIN chewable tablets are brownish, oval tablets that are scored in half so that the tablet can be divided in two equal parts.
- Both VETMEDIN CHEW and VETMEDIN chewable tablets manage the signs of congestive heart failure.
- You will notice the instructions for use in the VETMEDIN CHEW packaging to be slightly different than the instructions for the VETMEDIN chewable tablets. However, both VETMEDIN CHEW and VETMEDIN chewable tablets contain the same amount of pimobendan and the dose should be same.
Talk to your veterinarian if you have any questions about your dog’s congestive heart failure and the use of VETMEDIN CHEW.
Are there differences between VETMEDIN CHEW and VETMEDIN chewable tablets?
- Size – the VETMEDIN CHEW is smaller, about half the size of the FDA approved VETMEDIN chewable tablet.
- Strength - VETMEDIN CHEW is not available in 2.5 mg strength. The number and strength of VETMEDIN CHEW administered may be different than for VETMEDIN chewable tablets.
- Inactive ingredients – some of the additional ingredients that make up or hold the tablets together are different between the two.
- Packaging – VETMEDIN CHEW is packaged in cardboard boxes containing blister cards with 10 individually sealed tablets per card. VETMEDIN chewable tablets are packaged in bottles.
How do I give VETMEDIN CHEW to my dog?
VETMEDIN CHEW should be given to your dog in their mouth (orally) twice a day, about 12 hours apart as directed by your veterinarian. VETMEDIN CHEW should be given about 1 hour before feeding. If you have any questions about administration, please contact your veterinarian.
If a tablet is divided and only a half tablet is given to your dog, the remaining half should be returned to the open blister pocket and placed back in the cardboard box for storage. The remaining half tablet should be used within 3 days after opening the blister.
If your dog vomits after being given VETMEDIN CHEW, please contact your veterinarian and unless directed otherwise, do not give additional tablets again until the next scheduled dose.
What are some of the possible side effects of VETMEDIN Chew?
- VETMEDIN CHEW may cause side effects similar to those seen with VETMEDIN chewable tablets, even at the prescribed dose. Contact your veterinarian immediately if your dog develops a serious or concerning medical problem or side effect while taking VETMEDIN CHEW.
The most common side effects of pimobendan containing products administration are:
- poor appetite
- lethargy
- diarrhea
- difficulty breathing or coughing
- weakness or difficulty walking
- a rise in heart rate
- vomiting
There are other side effects which may occur with either VETMEDIN CHEW or VETMEDIN chewable tablets. For a complete list, ask your veterinarian.
To report a suspected adverse drug event (side effect) or a product quality problem contact Boehringer Ingelheim Animal Health USA Inc. at 1-888-637-4251. Adverse drug events and product quality problems may also be reported directly to FDA by completing the online form available at http://www.fda.gov/reportanimalaeor by requesting a hard copy of the form at 1-888-FDA-VETS.
What if my dog receives more VETMEDIN Chew than what is prescribed?
Contact your veterinarian immediately.
What else should I know about VETMEDIN CHEW?
VETMEDIN CHEW is not for use in humans.
You should keep VETMEDIN CHEW in a secure storage area out of the reach of children, as well as dogs, cats and other animals to prevent accidental ingestion or overdose.
If VETMEDIN CHEW is accidentally ingested by a person, contact a physician. It is important to show the treating physician a copy of the VETMEDIN CHEW package insert, label, or this information sheet. VETMEDIN CHEW is a non-sympathomimetic, non-glycoside inotropic drug with vasodilatative properties.
This information sheet contains a summary of important information about VETMEDIN CHEW. For more detailed information about VETMEDIN CHEW, talk with your veterinarian.
Storage Statement: VETMEDIN CHEW should not be stored at temperatures above 25°C (77°F).
Updated 02/2021
Marketing authorisation holder
Marketing authorisation holder in the UK
Boehringer Ingelheim Animal Health UK Ltd
Bracknell, RG12 8YS, UK.
Marketing authorisation holder in Ireland
Boehringer Ingelheim Vetmedica GmbH
55216 Ingelheim/Rhein, Germany
Manufacturer responsible for batch release
Lavet Pharmaceuticals Ltd.,
Kistarcsa, 2143 Batthyány u. 6., Hungary
Active substance
One chewable tablet contains:
Pimobendan: 1.25 mg
Pimobendan: 5 mg
Pimobendan: 10 mg
Brownish, oval, divisible tablet, scored on both sides.
The tablet can be divided into two equal parts.
Indications
For the treatment of canine congestive heart failure originating from dilated cardiomyopathy or valvular insufficiency (mitral and/or tricuspid valve regurgitation). (See also section “Dosage, routes and method of administration”).
For the treatment of dilated cardiomyopathy in the preclinical stage (asymptomatic with an increase in left ventricular end-systolic and end-diastolic diameter) in Doberman Pinschers following echocardiographic diagnosis of cardiac disease (see section “Special warnings” and “Precautions for use in animals”).
For the treatment of dogs with myxomatous mitral valve disease (MMVD) in the preclinical stage (asymptomatic with a systolic mitral murmur and evidence of increased heart size) to delay the onset of clinical symptoms of heart failure (see section “Special warnings” and “Special precautions for use in animals”).
Contraindications
Do not use pimobendan in hypertrophic cardiomyopathies or in diseases in which an improvement in cardiac output cannot be achieved for functional or anatomical reasons (e.g. aortic stenosis).
Since pimobendan is metabolised mainly via the liver, it should not be used in dogs with severe impairment of liver function (see also section “Pregnancy and lactation”). Do not use in cases of hypersensitivity to the active substance or to any of the excipients.
Adverse Reactions
In rare cases a slight positively chronotropic effect (rise in heart rate) and vomiting can occur. However, these effects are dose-dependent and can be avoided by reducing the dose.
In rare cases transient diarrhoea, anorexia or lethargy have been observed.
In rare cases, an increase in mitral valve regurgitation has been observed during chronic pimobendan treatment in dogs with mitral valve disease.
Although a relationship with pimobendan has not been clearly established, in very rare cases, signs of effects on primary haemostasis (petechiae on mucous membranes, subcutaneous haemorrhages) may be observed during treatment. These signs disappear when the treatment is withdrawn.
The frequency of adverse reactions is defined using the following convention:
- very common (more than 1 in 10 animals treated displaying adverse reactions)
- common (more than 1 but less than 10 animals in 100 animals treated)
- uncommon (more than 1 but less than 10 animals in 1,000 animals treated)
- rare (more than 1 but less than 10 animals in 10,000 animals treated)
- very rare (less than 1 animal in 10,000 animals treated, including isolated reports).
If you notice any side effects, even those not already listed in this package leaflet or you think that the medicine has not worked, please inform your veterinary surgeon.
Dosage, route and method of administration
Determine the bodyweight accurately before treatment to ensure correct dosage.
A dosage range of 0.2 mg to 0.6 mg pimobendan/kg body weight, divided into two doses daily, should be respected. The preferable daily dose is 0.5 mg pimobendan/kg body weight, divided into two doses daily.
This corresponds to:
One 1.25 mg chewable tablet in the morning and one 1.25 mg chewable tablet in the evening for a body weight of 5 kg.
One 5 mg chewable tablet in the morning and one 5 mg chewable tablet in the evening for a body weight of 20 kg.
One 10 mg chewable tablet in the morning and one 10 mg chewable tablet in the evening for a body weight of 40 kg.
Administration of pimobendan should take place approximately one hour before feeding.
Pimobendan may also be used in combination with a diuretic, e.g. furosemide or torasemide
Advice on correct administration
Do not exceed the recommended dosage.
To allow accurate dosing according to body weight, the chewable tablet can be halved along the designated score line.
Withdrawal period
Not applicable.
Special storage precautions
Keep out of the sight and reach of children.
Do not store above 25°C.
Divided tablets should be returned to the open blister pocket and placed back in the cardboard box.
Shelf life of the divided (halved) tablets after opening the blister: 3 days.
Do not use after the expiry date stated on the label after “EXP”. The expiry date refers to the last day of the month.
Warnings
Special warnings
Special warnings for each target species:
The product has not been tested in cases of asymptomatic DCM in Dobermans with atrial fibrillation or sustained ventricular tachycardia.
The product has not been tested in cases of asymptomatic myxomatous mitral valve disease in dogs with significant supraventricular and/or ventricular tachyarrhythmia.
Precautions
Special precautions for use in animals:
The blood glucose should be tested regularly during treatment in dogs with existing diabetes mellitus. For use in the preclinical stage of dilated cardiomyopathy (asymptomatic with an increase in left ventricular endsystolic and end-diastolic diameter), a diagnosis should be made by means of a comprehensive cardiac examination (incl. echocardiographic examination and possibly Holter monitoring).
For use in the preclinical stage of myxomatous mitral valve disease (stage B2, according to ACVIM consensus: asymptomatic with mitral murmur ≥3/6 and cardiomegaly due to myxomatous mitral valve disease), a diagnosis should be made by means of a comprehensive physical and cardiac examination which should include echocardiography or radiography where appropriate.
Monitoring of cardiac function and morphology is recommended in animals treated with pimobendan.
(See also section “Adverse Reactions”).
The chewable tablets are flavoured. In order to avoid any accidental ingestion, store tablets out of reach of animals.
Special precautions to be taken by the person administering the veterinary medicinal product to animals:
Wash hands after use.
To avoid accidental ingestion of the product by a child, divided or unused tablets should be returned to the open blister pocket and placed back in the cardboard box. In case of accidental ingestion, seek medical advice immediately and show the package leaflet or the label to the physician. Advice to doctors: accidental ingestion, especially by a child, may lead to the occurrence of tachycardia, orthostatic hypotension, flushing of the face and headaches.
Pregnancy and lactation:
Laboratory studies in rats and rabbits have not produced any evidence of teratogenic or foetotoxic effects. However, these studies have shown evidence of maternotoxic and embryotoxic effects at high doses, and have also shown that pimobendan is excreted into milk. The safety of the product has not been assessed in pregnant or nursing bitches. Use only according to the benefit/risk assessment by the responsible veterinarian.
Interaction with other medicinal products and other forms
of interaction:
In pharmacological studies no interaction between the cardiac glycoside ouabain (strophanthin) and pimobendan was observed. The pimobendan-induced increase in cardiac contractility is attenuated by the calcium antagonists verapamil and diltiazem and by the β- antagonist propranolol.
Overdose (symptoms, emergency procedures, antidotes):
An overdose may cause a positive chronotropic effect, vomiting, apathy, ataxia, heart murmurs or hypotension. In this situation, the dosage should be reduced and appropriate symptomatic treatment should be initiated.
In prolonged exposure (6 months) of healthy beagle dogs at 3 and 5 times the recommended dose, mitral valve thickening and left ventricular hypertrophy were observed in some dogs. These changes are of pharmacodynamic origin.
Incompatibilities:
None known.
Disposal of unused product or waste materials
Medicines should not be disposed of via wastewater or household waste. Ask your veterinary surgeon how to dispose of medicines no longer required. These measures should help to protect the environment.
Other information
For animal treatment only.
To be supplied only on veterinary prescription.
For any information about this veterinary medicinal product, please contact the local representative of the marketing authorization holder.
How Supplied
Heat sealed Aluminium// PVC/ Aluminium/ Polyamide blister containing 10 tablets.
Cardboard box with 5 blisters of 10 tablets (50 tablets).
Date on which the package leaflet was last approved
Nov 2019
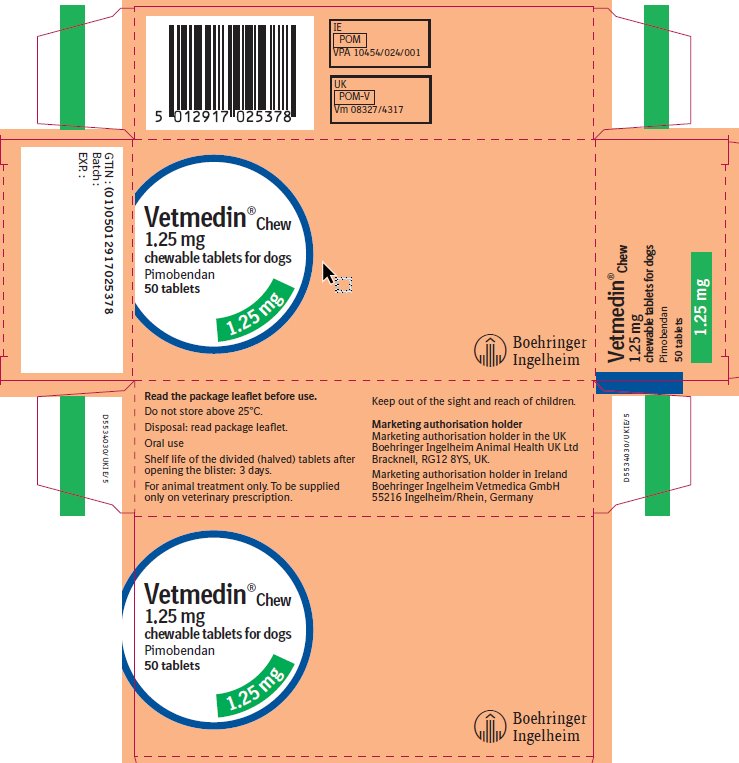
Principal Display Panel – 1.25 mg Display Carton 50 tablets
Vetmedin® Chew
1.25 mg
chewable tablets for dogs
Pimobendan
50 tablets
| VETMEDIN
CHEW
pimobendan tablet, chewable |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| VETMEDIN
CHEW
pimobendan tablet, chewable |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| VETMEDIN
CHEW
pimobendan tablet, chewable |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Boehringer Ingelheim Animal Health USA Inc. (007134091) |
Trademark Results [Vetmedin]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 VETMEDIN 79351045 not registered Live/Pending |
Boehringer Ingelheim Vetmedica GmbH 2022-07-26 |
 VETMEDIN 79056245 3580854 Live/Registered |
Boehringer Ingelheim Vetmedica GmbH 2008-06-09 |
 VETMEDIN 75794599 2429487 Live/Registered |
Boehringer Ingelheim Vetmedica GmbH 1999-09-08 |
 VETMEDIN 74051204 not registered Dead/Abandoned |
Boehringer Ingelheim Vetmedica GmbH 1990-04-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.