BABYGANICS ALCOHOL-FREE FOAMING HAND SANITIZER- benzalkonium chloride liquid
BabyGanics Alcohol-Free Foaming Hand Sanitizer by
Drug Labeling and Warnings
BabyGanics Alcohol-Free Foaming Hand Sanitizer by is a Otc medication manufactured, distributed, or labeled by KAS Direct LLC dba BabyGanics. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
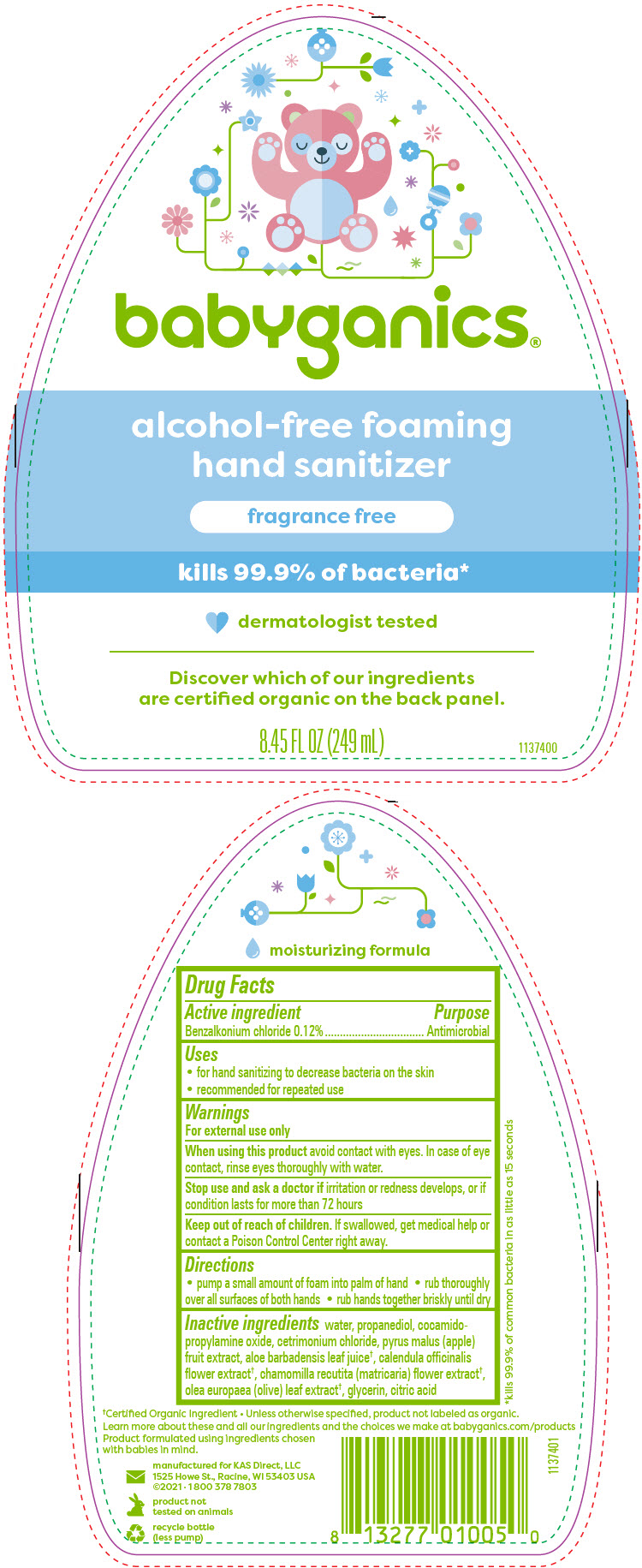
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
-
Inactive ingredients
water, propanediol, cocamidopropylamine oxide, cetrimonium chloride, pyrus malus (apple) fruit extract, aloe barbadensis leaf juice1, calendula officinalis flower extract1, chamomilla recutita (matricaria) flower extract1, olea europaea (olive) leaf extract1, glycerin, citric acid
- 1 Certified Organic Ingredient Unless otherwise specified, product not labeled as organic.
- PRINCIPAL DISPLAY PANEL - 249 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
BABYGANICS ALCOHOL-FREE FOAMING HAND SANITIZER
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 59062-3001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.12 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) PROPANEDIOL (UNII: 5965N8W85T) COCAMIDOPROPYLAMINE OXIDE (UNII: M4SL82J7HK) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) APPLE (UNII: B423VGH5S9) ALOE VERA LEAF (UNII: ZY81Z83H0X) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) CHAMOMILE (UNII: FGL3685T2X) OLIVE OIL (UNII: 6UYK2W1W1E) GLYCERIN (UNII: PDC6A3C0OX) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 59062-3001-2 250 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2022 2 NDC: 59062-3001-5 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2022 12/31/2027 3 NDC: 59062-3001-1 473 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 06/01/2022 12/31/2027 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug 505G(a)(3) 06/01/2022 Labeler - KAS Direct LLC dba BabyGanics (002764605)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.