ALBUTEROL SULFATE solution
Albuterol Sulfate by
Drug Labeling and Warnings
Albuterol Sulfate by is a Prescription medication manufactured, distributed, or labeled by Cardinal Health 107, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
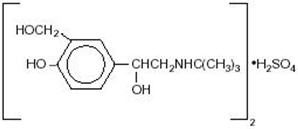
Albuterol sulfate inhalation solution is a relatively selective beta 2-adrenergic bronchodilator (see CLINICAL PHARMACOLOGY section below). Albuterol sulfate, the racemic form of albuterol, has the chemical name α 1-[( tert-Butylamino)methyl]-4-hydroxy- m-xylene-α,α'-diol sulfate (2:1) (salt), and the following structural formula:
Albuterol sulfate has a molecular weight of 576.70 and the molecular formula (C 13H 21NO 3) 2 H 2SO 4. Albuterol sulfate is a white or practically white powder, freely soluble in water and slightly soluble in alcohol.
The World Health Organization recommended name for albuterol base is salbutamol.
Albuterol sulfate inhalation solution 0.083% requires no dilution before administration.
Each milliliter of Albuterol Sulfate Inhalation Solution 0.083% contains 0.83 mg of albuterol (as 1 mg of albuterol sulfate) in an isotonic, sterile, aqueous solution containing sodium chloride; sulfuric acid is used to adjust the pH to between 3 and 5. Albuterol Sulfate Inhalation Solution 0.083% contains no sulfiting agents or preservatives.
Albuterol sulfate inhalation solution is a clear, colorless to light yellow solution.
-
CLINICAL PHARMACOLOGY
The prime action of beta-adrenergic drugs is to stimulate adenyl cyclase, the enzyme which catalyzes the formation of cyclic-3',5'-adenosine monophosphate (cyclic AMP) from adenosine triphosphate (ATP). The cyclic AMP thus formed mediates the cellular responses. In vitro studies and in vivo pharmacologic studies have demonstrated that albuterol has a preferential effect on beta 2-adrenergic receptors compared with isoproterenol. While it is recognized that beta 2-adrenergic receptors are the predominant receptors in bronchial smooth muscle, 10% to 50% of the beta-receptors in the human heart may be beta 2-receptors. The precise function of these receptors, however, is not yet established. Albuterol has been shown in most controlled clinical trials to have more effect on the respiratory tract in the form of bronchial smooth muscle relaxation than isoproterenol at comparable doses while producing fewer cardiovascular effects. Controlled clinical studies and other clinical experience have shown that inhaled albuterol, like other beta-adrenergic agonist drugs, can produce a significant cardiovascular effect in some patients, as measured by pulse rate, blood pressure, symptoms, and/or electrocardiographic changes.
Albuterol is longer acting than isoproterenol in most patients by any route of administration because it is not a substrate for the cellular uptake processes for catecholamines nor for catechol-O-methyl transferase.
Studies in asthmatic patients have shown that less than 20% of a single albuterol dose was absorbed following IPPB (intermittent positive-pressure breathing) or nebulizer administration; the remaining amount was recovered from the nebulizer and apparatus and expired air. Most of the absorbed dose was recovered in the urine 24 hours after drug administration. Following a 3 mg dose of nebulized albuterol, the maximum albuterol plasma level at 0.5 hour was 2.1 ng/mL (range 1.4 to 3.2 ng/mL). There was a significant dose-related response in FEV 1 (forced expiratory volume in one second) and peak flow rate.
It has been demonstrated that following oral administration of 4 mg albuterol, the elimination half-life was five to six hours.
Animal studies show that albuterol does not pass the blood-brain barrier. Recent studies in laboratory animals (minipigs, rodents, and dogs) recorded the occurrence of cardiac arrhythmias and sudden death (with histologic evidence of myocardial necrosis) when beta-agonists and methylxanthines were administered concurrently. The significance of these findings when applied to humans is currently unknown.
In controlled clinical trials, most patients exhibited an onset of improvement in pulmonary function within 5 minutes as determined by FEV 1. FEV 1 measurements also showed that the maximum average improvement in pulmonary function usually occurred at approximately 1 hour following inhalation of 2.5 mg of albuterol by compressor-nebulizer, and remained close to peak for 2 hours. Clinically significant improvement in pulmonary function (defined as maintenance of a 15% or more increase in FEV 1 over baseline values) continued for 3 to 4 hours in most patients and in some patients continued up to 6 hours.
In repetitive dose studies, continued effectiveness was demonstrated throughout the three-month period of treatment in some patients.
Published reports of trials in asthmatic children aged 3 years or older have demonstrated significant improvement in either FEV 1 or PEFR within 2 to 20 minutes following single dose of albuterol inhalation solution. An increase of 15% or more in baseline FEV 1 has been observed in children aged 5 to 11 years up to 6 hours after treatment with doses of 0.10 mg/kg or higher of albuterol inhalation solution. Single doses of 3, 4, or 10 mg resulted in improvement in baseline PEFR that was comparable to extent and duration to a 2 mg dose, but doses above 3 mg were associated with heart rate increases of more than 10%.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
As with other inhaled beta-adrenergic agonists, albuterol sulfate inhalation solution can produce paradoxical bronchospasm, which can be life threatening. If it occurs, the preparation should be discontinued immediately and alternative therapy instituted.
Fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs and with the home use of nebulizers. It is, therefore, essential that the physician instruct the patient in the need for further evaluation, if his/her asthma becomes worse. In individual patients, any beta 2-adrenergic agonist, including albuterol solution for inhalation, may have a clinically significant cardiac effect.
Immediate hypersensitivity reactions may occur after administration of albuterol as demonstrated by rare cases of urticaria, angioedema, rash, bronchospasm, and oropharyngeal edema.
-
PRECAUTIONS
General
Albuterol, as with all sympathomimetic amines, should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias and hypertension, in patients with convulsive disorders, hyperthyroidism or diabetes mellitus and in patients who are unusually responsive to sympathomimetic amines.
Large doses of intravenous albuterol have been reported to aggravate pre-existing diabetes mellitus and ketoacidosis. As with other beta-agonists, inhaled and intravenous albuterol may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease is usually transient, not requiring supplementation.
Repeated dosing with 0.15 mg/kg of albuterol inhalation solution in children aged 5 to 17 years who were initially normokalemic has been associated with an asymptomatic decline of 20% to 25% in serum potassium levels.
Information for Patients
The action of albuterol sulfate inhalation solution may last up to six hours, and therefore it should not be used more frequently than recommended. Do not increase the dose or frequency of medication without medical consultation. If symptoms get worse, medical consultation should be sought promptly. While taking albuterol sulfate inhalation solution, other anti-asthma medicines should not be used unless prescribed.
Drug compatibility (physical and chemical), efficacy, and safety of albuterol sulfate inhalation solution when mixed with other drugs in a nebulizer have not been established.
See illustrated " Patient's Instructions for Use."
Drug Interactions
Other sympathomimetic aerosol bronchodilators or epinephrine should not be used concomitantly with albuterol.
Albuterol should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, since the action of albuterol on the vascular system may be potentiated.
Beta-receptor blocking agents and albuterol inhibit the effect of each other.
Carcinogenesis, Mutagenesis, and Impairment of Fertility
Albuterol sulfate caused a significant dose-related increase in the incidence of benign leiomyomas of the mesovarium in a 2-year study in the rat, at oral doses of 2, 10, and 50 mg/kg corresponding to 10, 50 and 250 times, respectively, the maximum nebulization dose for a 50 kg human. In another study, this effect was blocked by the coadministration of propranolol. The relevance of these findings to humans is not known. An 18-month study in mice and a lifetime study in hamsters revealed no evidence of tumorigenicity. Studies with albuterol revealed no evidence of mutagenesis. Reproduction studies in rats revealed no evidence of impaired fertility.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Albuterol has been shown to be teratogenic in mice when given subcutaneously in doses corresponding to 1.25 times the human nebulization dose (based on a 50 kg human). There are no adequate and well-controlled studies in pregnant women. Albuterol should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. A reproduction study in CD-1 mice with albuterol (0.025, 0.25, and 2.5 mg/kg subcutaneously, corresponding to 0.125, 1.25 and 12.5 times the maximum human nebulization dose, respectively) showed cleft palate formation in 5 of 111 (4.5%) fetuses at 0.25 mg/kg and in 10 of 108 (9.3%) fetuses at 2.5 mg/kg. None were observed at 0.025 mg/kg. Cleft palate also occurred in 22 of 72 (30.5%) fetuses treated with 2.5 mg/kg isoproterenol (positive control). A reproduction study in Stride Dutch rabbits revealed cranioschisis in 7 of 19 (37%) fetuses at 50 mg/kg, corresponding to 250 times the maximum nebulization dose for a 50 kg human.
During worldwide marketing experience, various congenital anomalies, including cleft palate and limb defects, have been rarely reported in the offspring of patients being treated with albuterol. Some of the mothers were taking multiple medications during their pregnancies. No consistent pattern of defects can be discerned, and a relationship between albuterol use and congenital anomalies has not been established.
Labor and Delivery
Oral albuterol has been shown to delay preterm labor in some reports. There are presently no well-controlled studies that demonstrate that it will stop preterm labor or prevent labor at term. Therefore, cautious use of albuterol sulfate inhalation solution is required in pregnant patients when given for relief of bronchospasm so as to avoid interference with uterine contractibility.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because of the potential for tumorigenicity shown for albuterol in some animal studies, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and effectiveness of albuterol sulfate inhalation solution have been established in children 2 years of age or older. Use of albuterol sulfate inhalation solution in these age groups is supported by evidence from adequate and well-controlled studies of albuterol sulfate inhalation solution in adults; the likelihood that the disease course, pathophysiology, and the drug's effect in pediatric and adult patients are substantially similar; and published reports of trials in pediatric patients 3 years of age or older. The recommended dose for the pediatric population is based upon three published dose comparison studies of efficacy and safety in children aged 5 to 17 years, and on the safety profile in both adults and pediatric patients at doses equal to or higher than the recommended doses. The safety and effectiveness of albuterol sulfate inhalation solution in children below 2 years of age have not been established.
-
ADVERSE REACTIONS
Clinical Trial Experience
The results of clinical trials with albuterol sulfate inhalation solution in 135 patients showed the following side effects which were considered probably or possibly drug related:
Central Nervous System: tremors (20%), dizziness (7%), nervousness (4%), headache (3%), insomnia (1%).
Gastrointestinal: nausea (4%), dyspepsia (1%).
Ear, Nose and Throat: pharyngitis (<1%), nasal congestion (1%).
Cardiovascular: tachycardia (1%), hypertension (1%).
Respiratory: bronchospasm (8%), cough (4%), bronchitis (4%), wheezing (1%).
No clinically relevant laboratory abnormalities related to albuterol sulfate inhalation solution administration were determined in these studies.
In comparing the adverse reactions reported for patients treated with albuterol sulfate inhalation solution with those of patients treated with isoproterenol during clinical trials of three months, the following moderate to severe reactions, as judged by the investigators, were reported. This table does not include mild reactions.
Percent Incidence of Moderate to Severe Adverse Reactions Reaction Albuterol
N=65Isoproterenol
N=65- * The finding of no arrhythmias and no palpitations after albuterol administration in this clinical study should not be interpreted as indicating that these adverse effects cannot occur after the administration of inhaled albuterol.
- † In most cases of bronchospasm, this term was generally used to describe exacerbations in the underlying pulmonary disease.
Central Nervous System
Tremors
10.7%
13.8%
Headache
3.1%
1.5%
Insomnia
3.1%
1.5%
Cardiovascular
Hypertension
3.1%
3.1%
Arrythmias
0%
3%
*Palpitation
0%
22%
Respiratory
†Bronchospasm
15.4%
18%
Cough
3.1%
5%
Bronchitis
1.5%
5%
Wheeze
1.5%
1.5%
Sputum Increase
1.5%
1.5%
Dyspnea
1.5%
1.5%
Gastrointestinal
Nausea
3.1%
0%
Dyspepsia
1.5%
0%
Systemic
Malaise
1.5%
0%
Post-Marketing Experience
Cases of urticaria, angioedema, rash, bronchospasm, hoarseness, oropharyngeal edema, and arrhythmias (including atrial fibrillations, supraventricular tachycardia, extrasystoles) and metabolic acidosis have been reported after the use of albuterol sulfate inhalation solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
-
OVERDOSAGE
Manifestations of overdosage may include seizures, anginal pain, hypertension, hypokalemia, tachycardia with rates up to 200 beats/min, and exaggeration of the pharmacological effects listed in ADVERSE REACTIONS. In isolated cases in children 2 to 12 years of age, tachycardia with rates >200 beats/min has been observed.
The oral LD 50 in rats and mice was greater than 2,000 mg/kg. The inhalational LD 50 could not be determined.
There is insufficient evidence to determine if dialysis is beneficial for overdosage of albuterol inhalation solution.
-
DOSAGE AND ADMINISTRATION
Adults and Children 2 to 12 Years of Age
The usual dosage for adults and for children weighing at least 15 kg is 2.5 mg of albuterol (one vial) administered three to four times daily by nebulization. Children weighing < 15 kg who require < 2.5 mg/dose (i.e., less than a full vial) should use albuterol inhalation solution, 0.5% instead of albuterol inhalation solution, 0.083%. More frequent administration or higher doses are not recommended. To administer 2.5 mg of albuterol, administer the entire contents of one sterile unit dose vial (3 mL of 0.083% inhalation solution) by nebulization. The flow rate is regulated to suit the particular nebulizer so that albuterol inhalation solution will be delivered over approximately 5 to 15 minutes.
The use of albuterol sulfate inhalation solution can be continued as medically indicated to control recurring bouts of bronchospasm. During this time most patients gain optimum benefit from regular use of the inhalation solution.
If a previously effective dosage regimen fails to provide the usual relief, medical advice should be sought immediately, as this is often a sign of seriously worsening asthma which would require reassessment of therapy.
-
HOW SUPPLIED
Unit-dose plastic vial containing Albuterol Sulfate Inhalation Solution 0.083%, 2.5 mg/3 mL 1. Equivalent to 0.5 mL albuterol (as the sulfate) 0.5% (2.5 mg albuterol) diluted to 3 mL. Supplied in:
Overbagged with 5 x 3 mL vial in each bag, NDC: 55154-2132-5
WARNING: This Unit Dose package is not child resistant and is Intended for Institutional Use Only. Keep this and all drugs out of the reach of children.
- 1 (Potency expressed as albuterol, equivalent to 3 mg albuterol sulfate)
Storage
PROTECT FROM LIGHT. Store in pouch until time of use. Store between 2° and 25° C (36° and 77° F).
Rx only.
To report SUSPECTED ADVERSE REACTIONS, contact American Health Packaging at 1-800-707-4621 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
- SPL UNCLASSIFIED SECTION
-
Patient's Instructions for Use
Albuterol Sulfate Inhalation Solution 0.083%2
Note: This is a unit-dose vial. No dilution is required.Read complete instructions carefully before using.
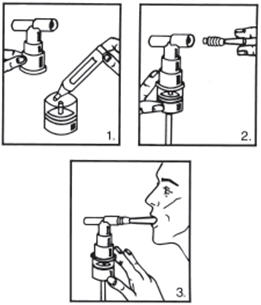
- 1. Remove the vial from the foil pouch.
- 2. Twist the cap completely off the vial and squeeze the contents into the nebulizer reservoir (Figure 1).
- 3. Connect the nebulizer reservoir to the mouthpiece or face mask (Figure 2).
- 4. Connect the nebulizer to the compressor.
- 5. Sit in a comfortable, upright position; place the mouthpiece in your mouth (Figure 3) (or put on the face mask); and turn on the compressor.
- 6. Breathe as calmly, deeply and evenly as possible until no more mist is formed in the nebulizer chamber (about 5 to 15 minutes). At this point, the treatment is finished.
- 7. Clean the nebulizer (see manufacturer's instructions).
Note: Use only as directed by your physician. More frequent administration or higher doses are not recommended.
Store Albuterol Sulfate Inhalation Solution 0.083%2 between 2° and 25° C (36° and 77° F). Store in pouch until time of use.
ADDITIONAL INSTRUCTIONS: ______________________________________________________________________________________
To report SUSPECTED ADVERSE REACTIONS, contact American Health Packaging at 1-800-707-4621 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Manufactured by:
The Ritedose Corporation
Columbia, SC 29203Distributed by:
American Health Packaging
Columbus, OH 43217Distributed By:
Cardinal Health
Dublin, OH 43017
L58112600824
RPIN0101
September 2018
- 2 (Potency expressed as albuterol, equivalent to 3 mg albuterol sulfate).
-
Package/Label Display Panel
NDC: 55154-2132-5
ALBUTEROL SULFATE INHALATION
SOLUTION, 0.083%* 2.5 mg/3 mL*
5 X 3 mL STERILE UNIT-DOSE VIALS
EACH IN A FOIL POUCH.
-
INGREDIENTS AND APPEARANCE
ALBUTEROL SULFATE
albuterol sulfate solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55154-2132(NDC:60687-395) Route of Administration RESPIRATORY (INHALATION) Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALBUTEROL SULFATE (UNII: 021SEF3731) (ALBUTEROL - UNII:QF8SVZ843E) ALBUTEROL 2.5 mg in 3 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM CHLORIDE (UNII: 451W47IQ8X) SULFURIC ACID (UNII: O40UQP6WCF) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55154-2132-5 5 in 1 BAG 01/16/2023 1 1 in 1 POUCH 1 3 mL in 1 AMPULE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077839 01/16/2023 Labeler - Cardinal Health 107, LLC (118546603)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.