MUCUS RELIEF DM COUGH- dextromethorphan hbr, guaifenesin tablet, film coated
Mucus Relief DM Cough by
Drug Labeling and Warnings
Mucus Relief DM Cough by is a Otc medication manufactured, distributed, or labeled by Major Pharmaceuticals, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
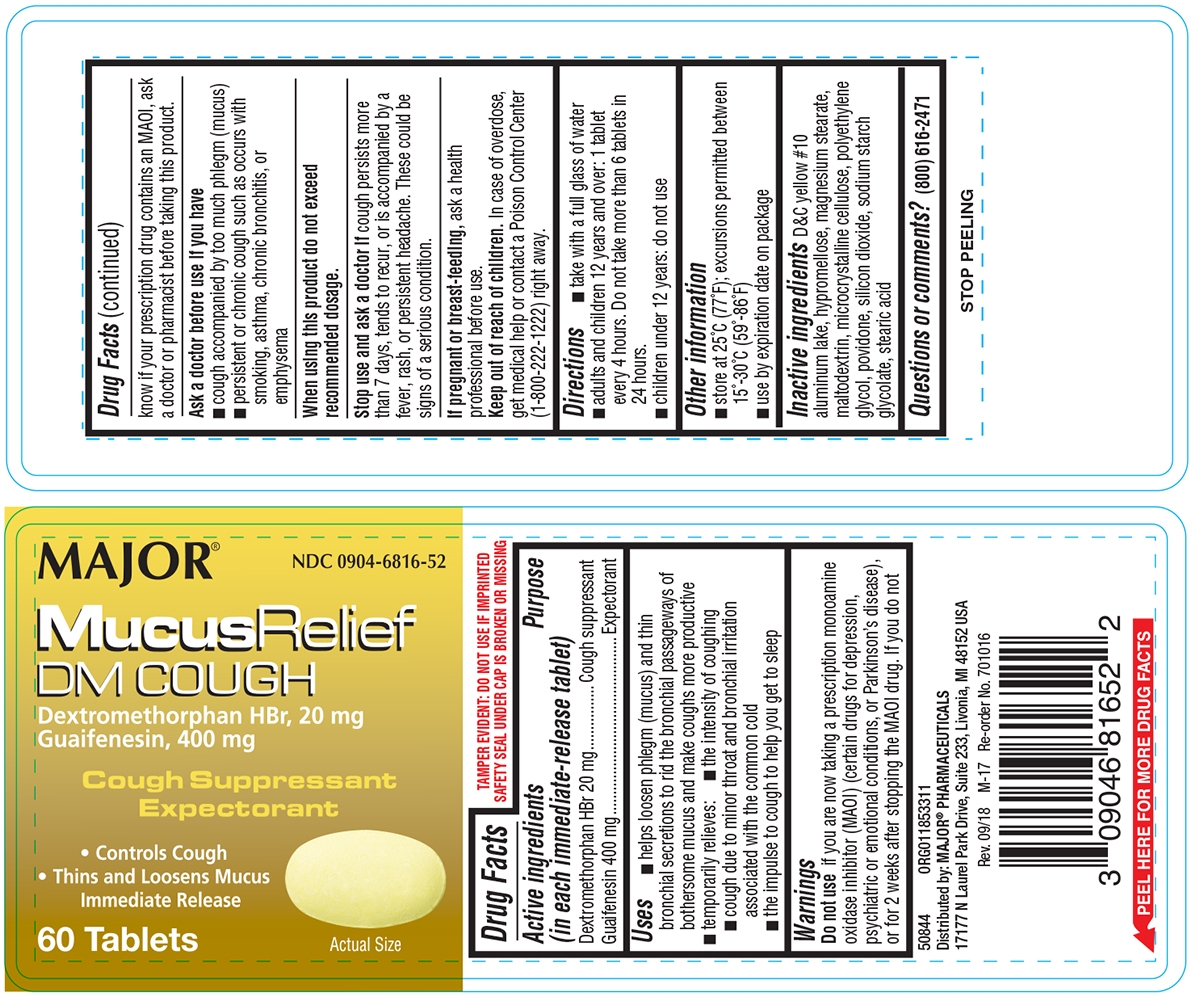
- Active ingredients (in each immediate-release tablet)
- Purpose
-
Uses
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive
- temporarily relieves:
- cough due to minor throat and bronchial irritation associated with the common cold
- the intensity of coughing
- the impulse to cough to help you get to sleep
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- cough accompanied by too much phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal Display Panel
MAJOR®
NDC: 0904-6816-52
MucusRelief
DM COUGHDextromethorphan HBr, 20 mg
Guaifenesin, 400 mgCough Suppressant
ExpectorantControls Cough
Thins and Loosens MucusImmediate Release
60 Tablets
Actual Size
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Distributed by: MAJOR® PHARMACEUTICALS
17177 N Laurel Park Drive, Suite 233, Livonia, MI 48152 USARev. 09/18 M-17 Re-order No. 701016
Major 44-533
-
INGREDIENTS AND APPEARANCE
MUCUS RELIEF DM COUGH
dextromethorphan hbr, guaifenesin tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0904-6816 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 20 mg GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 400 mg Inactive Ingredients Ingredient Name Strength D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POVIDONE (UNII: FZ989GH94E) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color YELLOW Score no score Shape OVAL Size 16mm Flavor Imprint Code 44;533 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0904-6816-46 30 in 1 BOTTLE; Type 0: Not a Combination Product 12/19/2018 2 NDC: 0904-6816-52 60 in 1 BOTTLE; Type 0: Not a Combination Product 12/19/2018 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 12/19/2018 Labeler - Major Pharmaceuticals (191427277) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(0904-6816) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(0904-6816) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(0904-6816) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(0904-6816) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(0904-6816)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.